Study to assess the Characteristics of Blood Pressure in Chronic Kidney Disease
Abhishek M Subramanya and Mohammed Fahad Khan*
Department of Nephrology, Manipal Hospitals, Bangalore, India
- *Corresponding Author:
- Mohammed Fahad Khan
Department of Nephrology,
Manipal Hospitals, Bangalore,
India,
E-mail: mohammedfahadkhan92@gmail.com
Received date: March 31, 2023, Manuscript No. IPJCEN-23-16209; Editor assigned date: April 03, 2023, PreQC No. IPJCEN-23-16209 (PQ); Reviewed date: April 14, 2023, QC No. IPJCEN-23-16209; Revised date: April 21, 2023, Manuscript No. IPJCEN-23-16209 (R); Published date: April 28, 2023, DOI: 10.36648/2472-5056.8.2.190.
Citation: Subramanya AM, Khan MF (2023) Study to Assess the Characteristics of Blood Pressure in Chronic Kidney Disease. J Clin Exp Nephrol Vol.8 No.2: 190.
Abstract
Background: Hypertension is an important modifiable risk factor for many complications. Hence, regulation of BP may substantially reduce the risk of cardiovascular events and slow the decline in kidney function. Hypertension is a major risk factor in the progression of Chronic Kidney Disease (CKD). Hypertension occurs in 86% of patients with CKD however, the proportion of patients who are able to maintain Blood Pressure (BP) control of <130⁄80 mmHg remains poor.
Objective: To assess the characteristics of blood pressure in chronic kidney disease.
Materials and Methods: A cross sectional study was conducted at a tertiary care centre in South India from 1st of January 2016 to 31st of July 2017. A total of 124 cases were included in the study.
Results: In our study 10.5% were in the age group <30 years. We reported that 33.1% had stage 3 CKD, 35.5% had stage 4 CKD and 31.5% had stage 5 CKD. There was significant difference in mean office SBP and mean office DBP with increase in stage of CKD. Mean OSBP and ODBP was increasing with increase in stage of CKD.
Conclusion: Evaluation of ambulatory BP also showed a remarkable amount of overestimation (white coat HTN) and underestimation (masked HTN) of BP among patients with CKD. Hence, using ABPM can circumvent errors in measurement of BP and also helpful in proper management of HTN in CKD.
Keywords
Kidney disease; Blood pressure; Cardiovascular; Hypertension; CKD; Ambulatory BP
Introduction
Chronic Kidney Disease (CKD) is a global public-health problem. Despite widespread use of interventions to slow the progression of CKD the burden of end-stage renal disease in many industrialized countries remains significant [1,2]. The prevalence of hypertension in patients with CKD is considerably higher than that in general population and escalates with a decline in renal function [3]. Elevated Blood Pressure (BP) causes injury to blood vessels in the kidney and other organs through excessive mechanical and oxidative stresses which can lead to complications such as renal failure and cardiovascular disease [3,4].
Hypertension is an important modifiable risk factor for such CKD, therefore regulation of BP may substantially reduce the risk of cardiovascular events and slow the decline in kidney function [5,6]. Hypertension is a major risk factor in the progression of Chronic Kidney Disease (CKD). Hypertension occurs in 86% of patients with CKD however, the proportion of patients who are able to maintain Blood Pressure (BP) control of <130⁄80 mmHg remains poor [7]. To reduce the risks, recent guidelines recommend strict control of Blood Pressure (BP) to ≤ 130/80 in CKD patients with and without proteinuria [8,9].
However, a large proportion of CKD patients have inadequate BP control and the proportions vary from studies to studies [10,11]. Clinic BP is considered insufficient to diagnose HTN and monitor overall BP control because it does not correlate well with Ambulatory Blood Pressure Monitoring (ABPM), which encompasses white-coat or masked HTN. CKD is associated not only with an abnormal dipping pattern but also with white-coat or masked HTN [12].
Objective
To assess the characteristics of blood pressure in chronic kidney disease.
Materials and Methods
A cross sectional study was conducted at a tertiary hospital in South India between 1st of January 2016 to 31st of July 2017.
A total of 124 patients with CKD included based on the inclusion and exclusion criteria.
All cases diagnosed with chronic kidney disease aged above 18 years who were admitted in the Department of Medicine and Nephrology in the hospital were included in the study. Patients suffering from HIV, cirrhosis, transplant recipients, patients on dialysis, pregnant mothers were excluded in the study.
Patients with CKD diagnosed based on ‘KDIGO 2012 clinical practice guideline for the Evaluation and Management of CKD’ were selected. The history was elicited with special reference to symptoms of CKD, CHF, comorbid condition, duration of diagnosing CKD and hemodialysis. A simple questionnaire was completed by each patient at the time of the ABPM and the questionnaire collected information such as the time the patient went to bed the time the patient got up, night-time was defined as actual sleep time using the patient’s diary.
Patients underwent 24 hrs ABPM using a TM-2430 monitor. Cuff size was chosen based on arm circumference and the cuff fixed to the non-dominant arm. Three BP readings were obtained in the morning (7:00 to 10:00 am) concomitant with sphygmomanometric measurements to ensure that the mean of the two sets of values differed by, 5 mmHg. BP was recorded every 20 min from 7:00 am to 10:00 pm and every 30 min from 10:00 pm to 7:00 am. The daytime and night-time periods were derived from diaries recorded by the patients during ABPM.
Results
A total of 124 study subjects were included in the study and analyzed.
In our study 10.5% were in the age group <30 years, 65.38% were in the age group 31 to 60 years and 24.2% were in the age group >60 years. Majority of them were males 71% and 29% were females. In the study 14.5% were on one anti-hypertensive, 29.8% were on two anti-hypertensive, 40.3% were on three antihypertensive and 15.3% were on four anti-hypertensive. We report that 33.1% had stage 3 CKD, 35.5% had stage 4 CKD and 31.5% had stage 5 CKD. In the study 46% had diabetes mellitus and 54% did not have diabetes mellitus. We noted that 21% had proteinuria of 2+, 53.2% had proteinuria of 3+, 25.8% had proteinuria of 4+ (Table 1).
| Count | % | ||
|---|---|---|---|
| Age | <30 years | 13 | 10.50% |
| 31 to 60 years | 81 | 65.30% | |
| >60 years | 30 | 24.20% | |
| Gender | Female | 36 | 29.00% |
| Male | 88 | 71.00% | |
| Type 2 diabetes mellitus | No | 67 | 54.00% |
| Yes | 57 | 46.00% | |
| Number of antihypertensive use | 1 | 18 | 14.50% |
| 2 | 37 | 29.80% | |
| 3 | 50 | 40.30% | |
| 4 | 19 | 15.30% | |
| Body mass index | Underweight (<18.5) | 3 | 2.40% |
| Normal (18.5 to 22.9) | 33 | 26.60% | |
| Overweight (23 to 24.9) | 20 | 16.10% | |
| Obese (>25) | 68 | 54.80% | |
| Stages of CKD | Stage 3 | 41 | 33.10% |
| Stage 4 | 44 | 35.50% | |
| Stage 5 | 39 | 31.50% | |
| Proteinuria | 2+ | 26 | 21.00% |
| 3+ | 66 | 53.20% | |
| 4+ | 32 | 25.80% | |
Table 1: General profile of CKD subjects in the study.
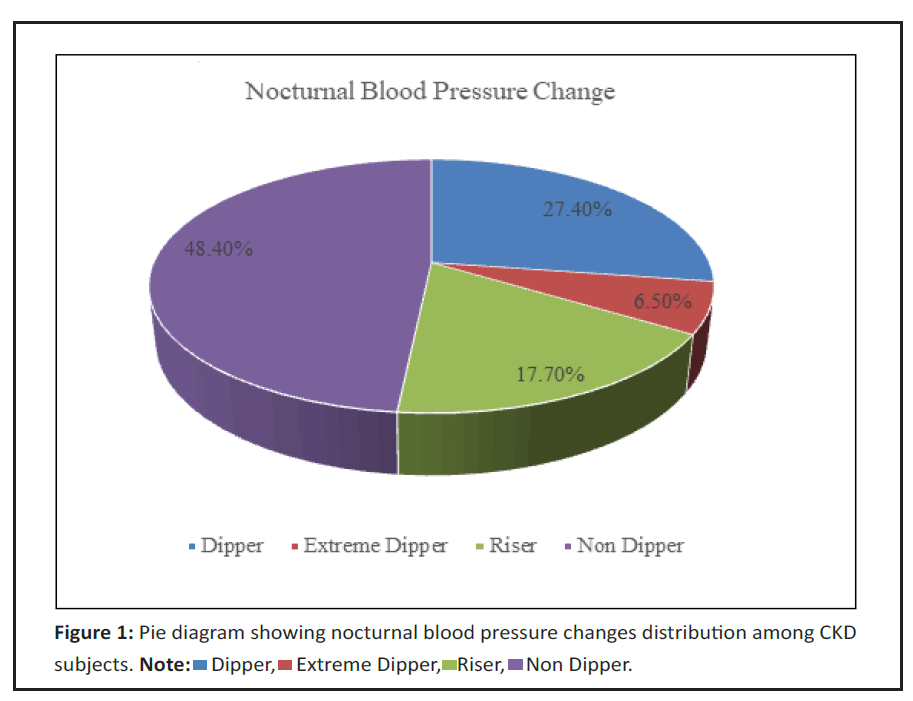
In the study 27.4% were dippers, 6.5% were extreme dippers, 17.7% were risers and 48.4% were non dippers. We also studied that 28.2% had controlled HTN, 29% had masked HTN, 35.5% had persistent HTN and 7.3% had white coat HTN (Figure 1).
In the study there was significant difference in mean OSBP and mean ODBP with increase in stage of CKD. Mean OSBP and ODBP was increasing with increase in stage of CKD (Table 2).
| OSBP | ODBP | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Stage of CKD | Stage 3 | 135 | 14.7 | 77.2 | 7.1 |
| Stage 4 | 138.5 | 12.8 | 77.7 | 7.2 | |
| Stage 5 | 146.7 | 12.7 | 82 | 6.4 | |
| P value | 0.001* | 0.004* | |||
| Note: *ANOVA test | |||||
Table 2: Comparison of mean office blood pressure with respect to stage of CKD.
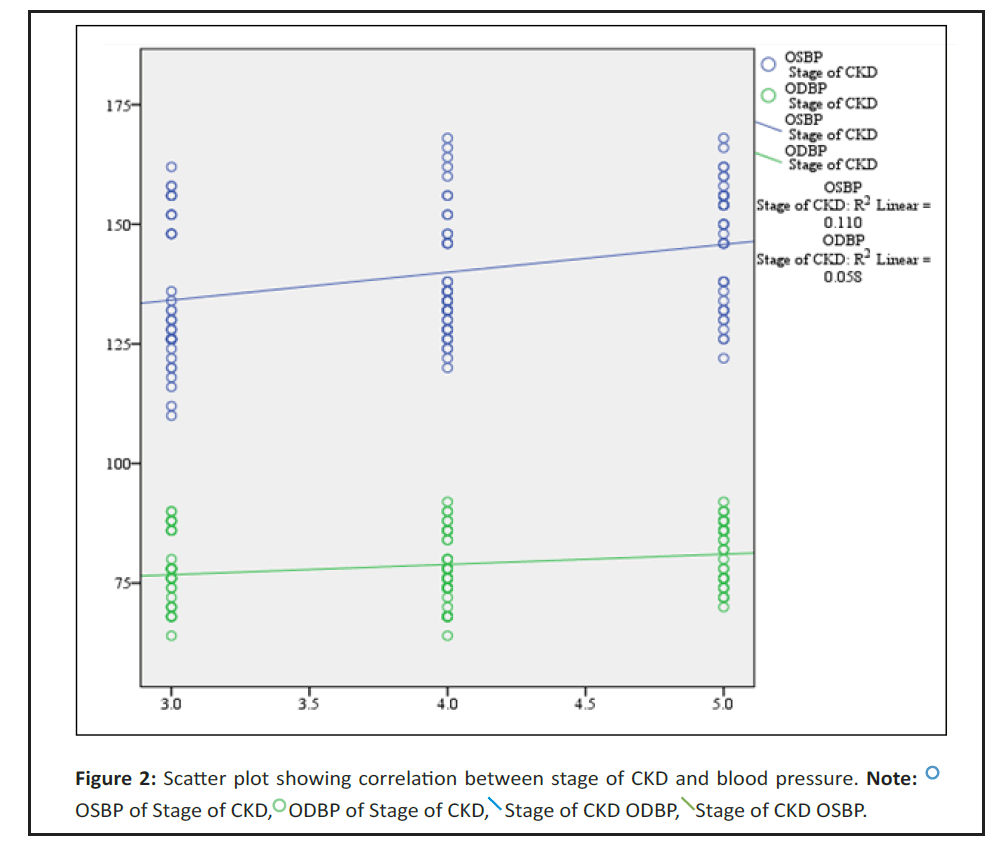
We noted that there is a significant positive correlation between stage of CKD and OSBP, ODBP. i.e. with increase in stage of CKD there was increase in OSBP, ODBP respectively and vs (Table 3 and Figure 2).
| Correlations | |||||
|---|---|---|---|---|---|
| Stage of CKD | OSBP | ODBP | |||
| Spearman's rho | Stage of CKD | Correlation coefficient | 1 | 0.346** | 0.209* |
| P value | . | <0.001* | 0.02* | ||
| N | 124 | 124 | 124 | ||
Table 3: Spearman’s correlation between stage of CKD and blood pressure.
Among stage 3 CKD subjects, 53.7% had controlled HTN, 12.2% had masked HTN, 12.2% had persistent HTN and 22% had white coat HTN, among stage 4 CKD subjects, 18.2% had controlled HTN, 50% had masked HTN, 31.8% had persistent HTN and 0% had white coat HTN and among stage 5 CKD subjects, 12.8% had controlled HTN, 23.1% had masked HTN, 64.1% had persistent HTN and 0% had white coat HTN. There was significant association between stage of CKD and type of HTN (Table 4).
| Stage of CKD | |||||||
|---|---|---|---|---|---|---|---|
| Stage 3 | Stage 4 | Stage 5 | |||||
| Count | Column N % | Count | Column N % | Count | Column N % | ||
| Type of HTN | Controlled HTN | 22 | 53.70% | 8 | 18.20% | 5 | 12.80% |
| Masked HTN | 5 | 12.20% | 22 | 50.00% | 9 | 23.10% | |
| Persistent HTN | 5 | 12.20% | 14 | 31.80% | 25 | 64.10% | |
| White Coat HTN | 9 | 22.00% | 0 | 0.00% | 0 | 0.00% | |
| Note: χ2=59.04, df=6, p<0.001* | |||||||
Table 4: Association between stage of CKD and type of HTN.
Discussion
In our study age of the patients varied from 18 to 80 years. The commonly affected age group in our study population was 31-60 years (61.38%). The mean age of patients was 48.8 ± 13.2 years. In a study by Gorostidi, et al., and Satoshi, et al. mean age was 61 ± 13.9, 60.8 ± 11.6 years respectively [11,12]. Prakash, et al. reported that the mean age was 47.5 ± 14.9 years and study observed that the mean age was 45.22 ± 15.2 years [13,14]. In our study the sex ratio was 2:4:1. Iimuro, et al., Yunkyu, et al. and Pogue, et al. reported that 62%, 58.4%, 62.1% were males respectively [15-17]. Compared to other western studies our study had more prevalence of male patients.
With respect to the stage of CKD in our patients, we reported that those in stage 3 were 33.1%, stage 4 was 35.5%, stage 5 was 31.5%. Satoshi, et al. [12] reported that their prevalence of CKD was 43.8%, 41.7%, 14.4% respectively in the three stages.
46% patients had diabetes in our study which is similar to the international data. Satoshi, et al. [12] published that 35.4% had diabetes and Singh, et al. [14] documented that 31.6% had diabetes. In our study diabetes was the main cause for CKD. In the study 21% had proteinuria of 2+, 53.2% had proteinuria of 3+ and 25.8% had proteinuria of 4+. Similar results were reported by Satoshi, et al. [12] where 87% patients had proteinuria.
In this study extreme dippers were 6.5%, dippers were 27.4%, non-dippers 48.4%, risers 17.7. In Otero, et al. [18] 4.7%, 47.2%, 42.3%, 5.9% were present in the respective groups. Since our’s is a tertiary care center prevalence of advanced stage of CKD is more when compared to other studies and hence prevalence of non-dippers and risers are relatively more when compared to other studies.
In our study mean OSBP of stage 3, 4, 5 is 135 ± 14.7, 138.5 ± 12.8, 146.7 ± 12.7 and mean ODBP 77.2 ± 7.1, 77.7 ± 7.2, 82 ± 6.4. We report that there is a significant positive correlation between stage of CKD, OSBP and ODBP i.e. with increase in stage of CKD there was increase in OSBP, ODBP respectively and vice versa. In Satoshi, et al. [12] OSBP of stage 3, 4, 5 is 129.7 ± 17.2, 132.7 ± 17.8, 137.8 ± 18.8 and mean ODBP 77.9 ± 11.3, 77.3 ± 11.5, 77.5 ± 12.1. Same pattern was also found in Satoshi, et al. [12].
In our study prevalence of controlled HTN was decreasing from 53.7% to 12.8% as stage of CKD progresses, prevalence of masked HTN increases from 12.2% to 23.1%-50% and prevalence of persistent HTN increases from 12.2% to 64.1%.There was significant association between stage of CKD and type of HTN. In Satoshi, et al. [12] as for the CKD stage, prevalence of controlled blood pressure decreased from 42.3% to 29.0% and that of persistent HTN rose from 21.7% to 36.1% with advancing CKD stage.
Our study goes to show the prevalence of hypertension in CKD and the characteristics of hypertension in these patients. It also demonstrates a positive correlation of hypertension with CKD and the increasing trend in its values with the progression of CKD. We also report the lack of nocturnal dipping in CKD patients which is as high as 66%. We also reported the increasing prevalence of masked hypertension, white coat hypertension in the various stages of CKD which could only be detected with ambulatory blood pressure monitoring, thereby highlighting its significance.
Conclusion
Our study showed that 24 hour ABPM provides a more reliable assessment of BP in patients with CKD. Evaluation of ambulatory BP also showed a remarkable amount of overestimation (white coat HTN) and underestimation (masked HTN) of BP among patients with CKD so by using ABPM we can circumvent errors in measurement of BP and displays its utility in appropriate management of HTN in CKD. We found that prevalence of persistent HTN was high in CKD population and increased in association with progression of CKD. Prevalence of none dipping and risers are more in advanced stages of CKD.
References
- Stenvinkel P (2010) Chronic kidney disease: A public health priority and harbinger of premature cardiovascular disease. J Intern Med 268: 456-467.
[Crossref], [Google Scholar], [Indexed]
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, et al. (2007) Prevalence of chronic kidney disease in the United States. JAMA 298: 2038-2047.
[Crossref], [Google Scholar], [Indexed]
- Portaluppi F, Boari B, Manfredini R (2004) Oxidative stress in essential hypertension. Curr Pharm Des 10: 1695-1698.
[Crossref], [Google Scholar], [Indexed]
- National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39: S1-S266.
[Indexed]
- Drawz PE, Abdalla M, Rahman M (2012) Blood pressure measurement: Clinic, home, ambulatory and beyond. Am J Kidney Dis 60: 449-462.
- Sarafidis PA, Khosla N, Bakris GL (2007) Antihypertensive therapy in the presence of proteinuria. Am J Kidney Dis 49: 12-26.
[Crossref], [Google Scholar], [Indexed]
- Sarafidis PA, Li S, Chen SC, Collins AJ, Brown WW, et al. (2008) Hypertension awareness, treatment and control in chronic kidney disease. Am J Med 121: 332-340.
[Crossref], [Google Scholar], [Indexed]
- Agarwal R, Nissenson AR, Battle D, Coyne DW, Trout JR, et al. (2003) Prevalence, treatment and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 115: 291-297.
[Crossref], [Google Scholar], [Indexed]
- Kidney Disease Improving Global Outcome (KDIGO) CKD work group (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1-150.
[Crossref], [Google Scholar]
- Muntner P, Anderson A, Charleston J, Chen Z, Ford V, et al. (2010) Hypertension awareness, treatment and control in adults with CKD: Results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 55: 441-451.
[Crossref], [Google Scholar], [Indexed]
- Gorostidi M, Sarafidis PA, de la Sierra A, Segura J, de la Cruz JJ, et al. (2013) Differences between office and 24-hour blood pressure control in hypertensive patients with CKD: A 5693 patient cross sectional analysis from Spain. Am J Kidney Dis 62: 285-294.
- Cunha C, Pereira S, Fernandes JC, Dias VP (2017) 24-hour ambulatory blood pressure monitoring in chronic kidney disease and its influence on treatment. Chinese General Practice 29: 161-169.
- Praksah S, Chibber SK, Prakash S, Pande DP, Joshi S, et al. (2005) Assessment of hypertension control in chronic kidney disease patients by ambulatory blood pressure monitoring. J Assoc Physicians India 53: 769-774.
[Indexed]
- Singh AK, Farag YMK, Mittal BV, Subramanian KK, Reddy S, et al. (2013) Epidemiology and risk factors of chronic kidney disease in India-results from the SEEK ( Screening and Early Evaluation of Kidney Disease) study. BMC Nephrol 14: 114.
[Crossref], [Google Scholar], [Indexed]
- Iimuro S, Imai E, Watanabe T, Nitta K, Akizawa T, et al. (2013) Clinical correlates of ambulatory BP monitoring among patients with CKD. Clin J Am Soc Nephrol 8: 721-730.
[Crossref], [Google Scholar], [Indexed]
- Oh YK, Chin HJ, Ahn SY, An JN, Lee JP, et al. (2017) Discrepancies in clinic and ambulatory blood pressure in Korean chronic kidney disease patients. J Korean Med Sci 32: 772-781.
[Crossref], [Google Scholar], [Indexed]
- Pogue V, Rahman M, Lipkowitz M, Toto R, Miller E, et al. (2009) Disparate estimates of hypertension control from ambulatory and clinic blood pressure measurements in hypertensive kidney disease. Hypertension 53: 20-27.
- Otero A, Dominguez SM, Castineria MC, Crespo JJ, Ferras A, et al. (2011) Alteration of the circadian blood pressure pattern in subjects with chronic kidney disease: The HYGIA project. Hypertension 29: e174.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
 OSBP of Stage of CKD,
OSBP of Stage of CKD, ODBP of Stage of CKD,
ODBP of Stage of CKD, Stage of CKD ODBP,
Stage of CKD ODBP,  Stage of CKD OSBP.
Stage of CKD OSBP.