Urinary Exosomal Aquaporin-2 Expression and the Efficacy of Tolvaptan in Advanced CKD Patients with Congestive Heart Failure: An Interim Report
Kenzo Kodaka, Emiko Kuribayashi Okuma, Shigeyuki Arai, Michito Nagura, Yoshifuru Tamura, Shigeru Shibata, Yoshihide Fujigaki and Shunya Uchida
DOI10.21767/2472-5056.100057
Kenzo Kodaka, Emiko Kuribayashi Okuma, Shigeyuki Arai, Michito Nagura, Yoshifuru Tamura, Shigeru Shibata, Yoshihide Fujigaki and Shunya Uchida*
Department of Internal Medicine, Teikyo University School of Medicine, 2-11-1 Kaga Itabashi, Tokyo 1738605, Japan
- *Corresponding Author:
- Shunya Uchida
Department of Internal Medicine, Teikyo University School of Medicine
2-11-1 Kaga Itabashi, Tokyo 1738605, Japan
Tel: +81339641211
Fax: +81339648942
E-mail: s_uchida@netjoy.ne.jp
Received date: March 05, 2018; Accepted date: March 23, 2018; Published date: April 02, 2018
Citation: Kodaka K, Okuma EK, Arai S, Nagura M, Tamura Y, et al. (2018) Urinary Exosomal Aquaporin-2 Expression and the Efficacy of Tolvaptan in Advanced CKD Patients with Congestive Heart Failure-an Interim Report. J Clin Exp Nephrol Vol 3:6. DOI: 10.21767/2472-5056.100057
Copyright: © 2018 Kodaka K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The efficacy of tolvaptan, a selective antagonist for vasopressin V2 receptor, on water diuresis has been reported in congestive heart failure (CHF) patients with chronic kidney disease (CKD). However, the mechanism of tolvaptan resistance is not well understood. In this study the effect of tolvaptan on urinary expression of exosomal aquaporin-2 (AQP-2) was examined in relation to effectiveness of tolvaptan.
Methods: We examined 13 CHF patients with advanced CKD (M:F=11:2, age 49 to 81, serum creatinine 1.7 to 10.4 mg/dL, eGFR 3.5 to 69 ml/min/1.73 m2, median 9.2) who were not under control with conventional treatment including furosemide therapy (40 to 80 mg orally). Tolvaptan of 7.5 mg/day was instituted and blood and urine parameters were measured as well as urinary exosomal AQP-2 and Na-K-2Cl cotransporter (NKCC2). Responders were defined as increase in urine volume greater than 500 ml/day after the treatment.
Results: Eight patients were diagnosed as responders who increased urine volume together with decrease in body weight within several days (p<0.05 vs. before treatment). Baseline data did not show the significant difference between both groups. Serum levels of creatinine and Na did not change over 1 week in either responders or non-responders. All responders showed a significant reduction in urinary exosomal AQP-2 after the treatment whereas non-responders showed rather increase in its expression. In contrast, urinary exosomal NKCC2 expression was unaltered in any case.
Conclusions: Tolvaptan exerts its diuretic action in more than half the CHF patients complicated with advanced CKD. The responsiveness corroborates well with urinary exosomal AQP2 expression. The predictor for resistance to tolvaptan needs further examination.
Keywords
Aquaporin-2; Tolvaptan; Exosome; Congestive heart failure; Chronic kidney disease
Introduction
In a broad cohort of Japanese patients with congestive heart failure (CHF), 70% of patients showed an estimated glomerular filtration rate (eGFR) of less than 60 ml/min 1.73 m2, that falls into chronic kidney disease (CKD) and that CKD was independently associated with long-term adverse outcomes of CHF patients [1].
Although loop diuretics, natriuretic agents, are used as a standard medication for CHF, the drug sometimes induces metabolic and electrolyte disorders and also exacerbates renal function by activation of the renin-angiotensin-aldosterone system and sympathetic nervous system, volume contraction, all resulting in decline in renal blood flow [2,3]. In fact, treatment with higher doses of loop diuretics was associated with the poor prognosis of the patients hospitalized due to CHF [4].
Tolvaptan is an aquaretic drug that preferentially excrete water rather than sodium by selectively antagonizing vasopressin V2 receptors (V2R), thus inhibiting the movement of aquaporin-2 (AQP-2) into the luminal membrane of the collecting duct cells [5,6] where AQP-2 mediates water transport [7,8]. In addition to standard therapy including natriuretic diuretics, tolvaptan could improve CHF, without serious adverse events in patients hospitalized with CHF [9]. In contrast to loop diuretics, tolvaptan did not activate the renin-angiotensin-aldosterone system and the sympathetic nervous system [10].
However, there are a certain number of patients showing resistance to tolvaptan among CHF patients and decrease in kidney function and higher age may affect the response to tolvaptan [11]. Excretion of AQP-2 from the renal collecting ducts into urine has been considered as a functional marker of the collecting duct [12]. Along this scenario, it is reported that efficacy of tolvaptan may be closely associated with urinary AQP-2 level in patients with CHF [13]. However, there is little data about the relationship between tolvaptan responsiveness and urinary excretion of AQP-2, especially exosomal AQP-2 in CHF patients with CKD.
In an attempt to investigate the mechanism of tolvaptan resistance, we tried to examine the expression of AQP-2 before and after tolvaptan treatment in CHF patients with advanced CKD and its correlation to conventional laboratory findings.
Methods
Subjects
13 CHF patients with CKD stage 3 or higher were enrolled after obtaining written informed consent. The patients were categorized as New York Heart Association functional class IV (NYHA IV) and considered resistant to conventional treatment with loop diuretics. Initial dose of tolvaptan was started at 7.5 mg/day and kept constant over 1 week. Volume of infusion was not changed and drinking was not restricted during this study period. The patients were defined as responders to tolvaptan when urine volume increased greater than 500 ml/day within several days after tolvaptan introduction.
The present study was approved by the institutional review board (IRB) in the Teikyo University Review Board #14-168 and was executed in accordance with the principle of the Helsinki Declaration. Before executing the study, written informed consent was obtained from the patient and the patient record and information were anonymized and de-identified prior to analysis.
Measurements
All clinically relevant measurements were examined before and after tolvaptan treatments. Body weight, urinary volume and blood and urine samples were collected to measure serum creatinine, serum Na, serum K, and urine osmolality. Adverse events after tolvaptan treatment were evaluated with special attention to hypernatremia. In addition, we examined the expression of AQP-2 in urine exosome before and 1 week after the administration of tolvaptan. The expression of sodium-potassium- chloride cotransporter (NKCC2), a specific luminal transporter in the thick ascending limb of Henle [14], was assessed in comparison.
The isolation of urinary proteins in the exosome fraction was described elsewhere [15]. Briefly, the exosomes were isolated from the urine samples using differential centrifugation. The urine was centrifuged at 1,500 g for 15 min to eliminate the cells and debris and at 17,000 g for 15 min, followed by ultracentrifugation at 200,000 g for 60 min. After removal of the supernatant, the pellets were suspended in 25 μL isolation solution. The suspension was then added to an equal volume of 2X sample buffer. These samples were heated at 60 for 15 min and then stored at -80°C until use.
It is appreciated that this fraction of urine is largely made up of excreted exosomes [16]. Then, gel electrophoresis and immunoblotting were performed. For quantitative comparison, applied volume of urine exosome fraction to SDS-PAGE was corrected by urine creatinine concentration. Each sample was loaded with the same amount of creatinine (4 mg per lane) to SDS-PAGE with 4-20% polyacrylamide slab gels (e-PAGEL; ATTO Corporation, Tokyo, Japan).
We obtained peptide-derived polyclonal antibody to goat AQP-2 from Santa Cruz Biotechnology (CA, USA). NKCC2 antibody was raised against a synthetic peptide corresponding to a sequence at the NH2-SDSTDPPHYEETSFGDEAQNRLKC-COOH reported previously [14]. After transfer by wet-type method to PVDF membranes (ATTO Corporation, Tokyo, Japan), membranes were blocked with 5% non-fat dry milk and 0.05% Tween 20 in TBS overnight at 4°C. Then, blots were incubated for 1 h at room temperature with anti-AQP-2 (1:1000) or anti-NKCC2 (1:1000), respectively in IMMUNOSHOT 1 solution (COSMO BIO Co. Ltd., Tokyo, Japan).
The membranes were incubated with the respective secondary antibody. For visualizing AQP-2, Alexa Fluor 647 donkey anti-goat IgG (Molecular probes, Eugene, OR, USA) was incubated in IMMUNOSHOT 2 solution (1:8000) or for NKCC2, Alexa Fluor 546 goat anti-rabbit IgG (Molecular probes, Eugene, OR, USA) was used in IMMUNOSHOT 2 solution (1:8000) for 1 h at room temperature. Blots were washed three times with TBS/ Tween, and then visualized using laser detection (Typhoon Trio, GE healthcare, UK). Image J software was used to quantify the intensity of the reactive bands.
Statistics
Values for categorical variables are given as number (or percentage) and values for continuous variables are given as mean ± standard deviation (SD). The proportion of categorical data was assessed by Fisher’s exact test. Time-dependent changes in parameters were evaluated by paired t test for comparisons of before and after the treatment. The difference between two groups at the same time point was compared by unpaired t test. Statistical analyses were undertaken using SPSS statistics for Windows version 22 (IBM Corp, Armonk, NY, USA). A p value less than 0.05 was considered statistically significant.
Results
Enrolled patients were 13 CHF patients with advanced CKD (M:F=11: 2, age 49 to 81, serum creatinine 1.7 to 10.4 mg/dL, eGFR 3.5 to 69 ml/min/1.73 m2, median 9.2). Eight patients (62%) were regarded as responders to tolvaptan because urine volume increased more than 500 ml/day after tolvaptan treatment whereas non-responders accounted for 5 out of 13 patients (38%).
A comparison of clinical characteristics for responders and non-responders to tolvaptan is shown in (Table 1). Age, sex, eGFR, body weight, urine volume, serum creatinine, serum Na, urine creatinine concentration, urine urea nitrogen concentration, urine Na concentration, and urine osmolality before tolvaptan treatment were not significantly different between responders and non-responders.
| Responders | Non-responders | p value | |
|---|---|---|---|
| Cases (n) | 8 | 5 | |
| Age (years) | 64 ± 13 | 68 ± 19 | 0.67 |
| Male: Female | 0:8 | 2:3 | 0.13 ¶ |
| eGFR (ml/min/1.73 m2) | 13.7 ± 10.5 | 18.9 ± 28.4 | 0.64 |
| Body weight (kg) | 64.9 ± 18.4 | 60.5 ± 15.4 | 0.66 |
| Urine volume (ml) | 1,010 ± 506 | 1,000 ± 484 | 0.92 |
| Serum Cr (mg/dL) | 6.0 ± 4.1 | 5.6 ± 4.6 | 0.80 |
| Serum Na (mEq/L) | 133.5 ± 7.9 | 134.2 ± 9.1 | 0.89 |
| Urine Cr (mg/dL) | 35.6 ± 19.1 | 62.5 ± 38.5 | 0.24 |
| Urine UN (mg/dL) | 201 ± 131 | 308 ± 190 | 0.36 |
| Urine Na (mEq/L) | 64 ± 20 | 78 ± 41 | 0.45 |
| Urine osmolality (mOsm/kgH2O) | 280 ± 52 | 317 ± 160 | 0.55 |
| eGFR: Estimated glomerular filtration rate; Cr: Creatinine; Na: Sodium; UN: Urea nitrogen; ¶: Fisher’s exact test | |||
Table 1: Clinical characteristics and laboratory findings at the baseline in responders and non-responders after tolvaptan treatment.
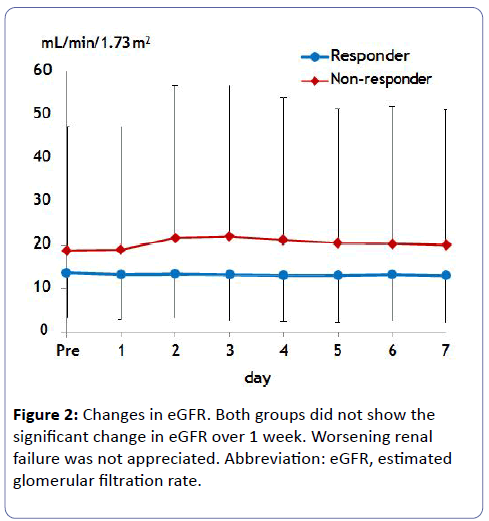
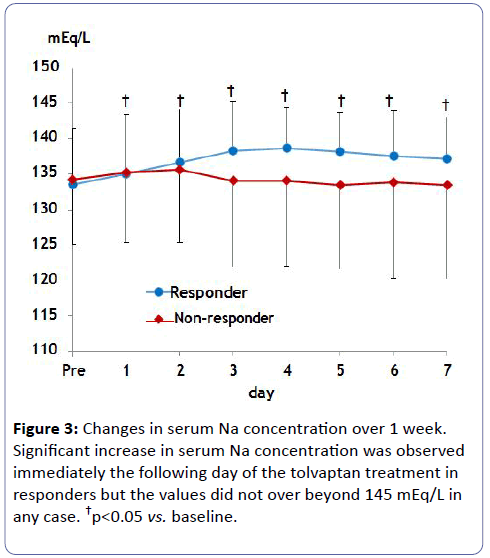
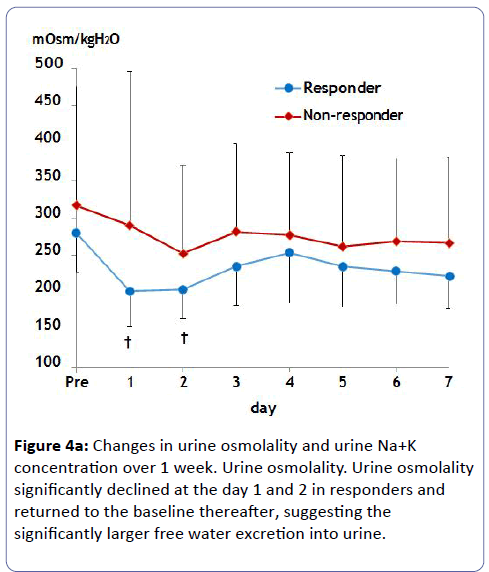
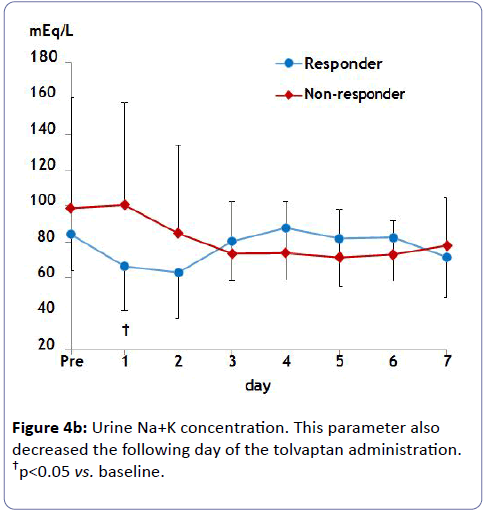
Estimated GFR did not change in either group, which avoided worsening renal function (Figure 2). Serum Na concentration did not show a significant increase over 1 week probably due to small initial dosing in either case (Figure 3). Of note, urine osmolality and urine Na+K concentration significantly decreased 1 to 2 days after tolvaptan treatment only in responders, suggesting the increase in free water clearance (Figure 4).
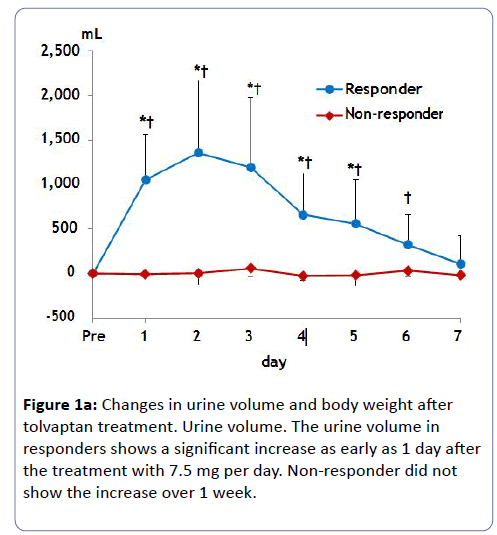
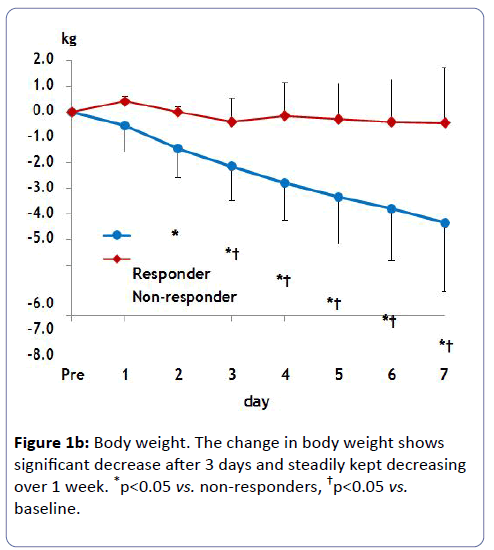
Urine volume in responders peaked at 1,000 to 1,500 ml/day within several days after tolvaptan treatment (Figure 1a). The average body weight loss in responders was 4.3 kg until 7 days (Figure 1b). Non-responders did not change urinary volume or body weight after tolvaptan treatment over 1 week.
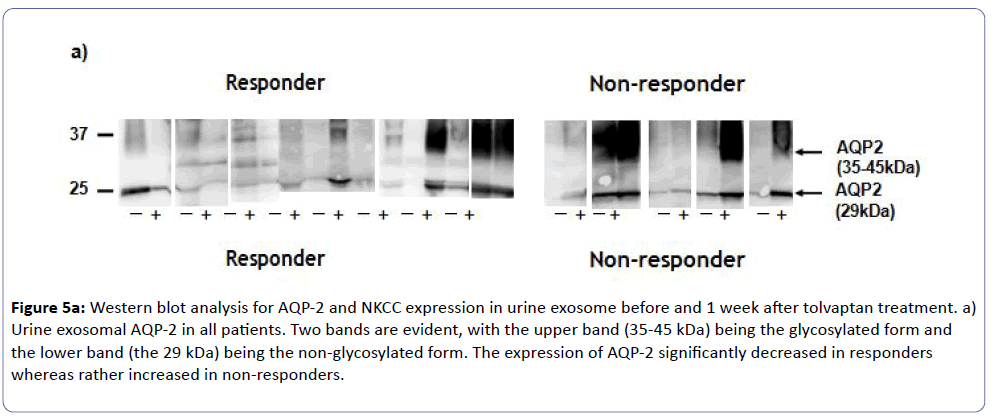
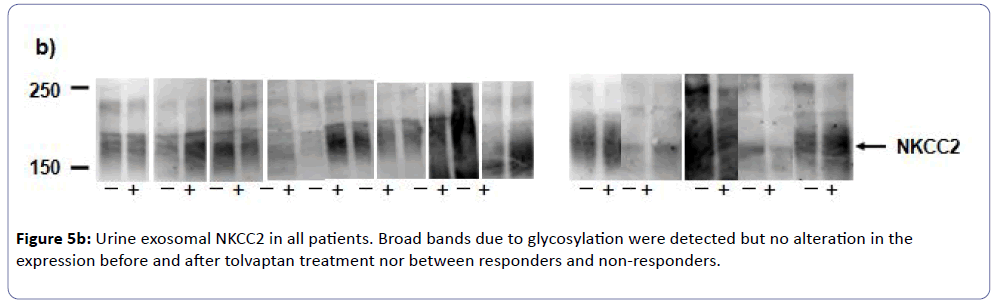
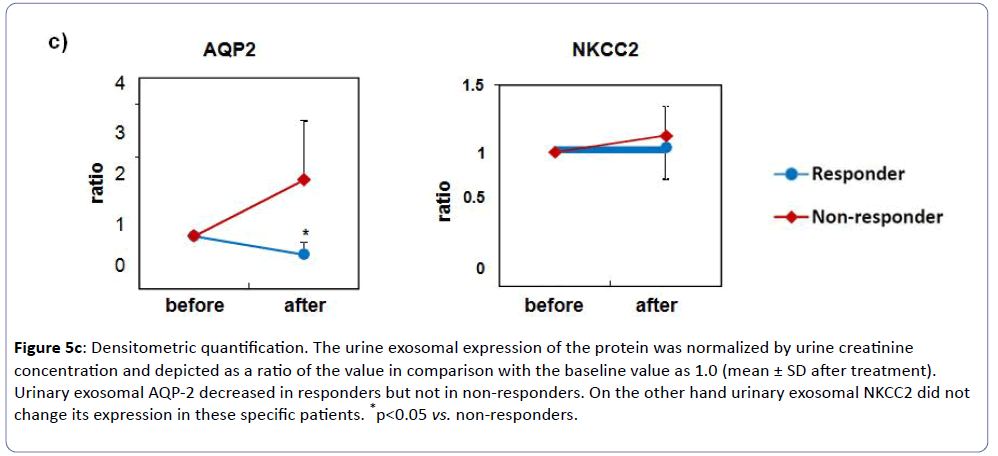
Western blot revealed a 29-kDa band in the membrane fractions from urine, which corresponded to AQP-2 protein by immunoblotting. As shown in (Figure 5a), all responders showed significant reduction of urinary exosomal AQP-2 expression after tolvaptan treatment, whereas those in non-responders rather increased (Figure 5c). On the other hand, western blot showed no change of an abundance of 150-kDa bands for NKCC2 in all subjects before and after the tolvaptan treatment (Figures 5b and 5c). These findings were consistent with the effectiveness of tolvaptan in CHF complicated with advanced CKD.
Figure 5a: Western blot analysis for AQP-2 and NKCC expression in urine exosome before and 1 week after tolvaptan treatment. a) Urine exosomal AQP-2 in all patients. Two bands are evident, with the upper band (35-45 kDa) being the glycosylated form and the lower band (the 29 kDa) being the non-glycosylated form. The expression of AQP-2 significantly decreased in responders whereas rather increased in non-responders.
Figure 5c: Densitometric quantification. The urine exosomal expression of the protein was normalized by urine creatinine concentration and depicted as a ratio of the value in comparison with the baseline value as 1.0 (mean ± SD after treatment). Urinary exosomal AQP-2 decreased in responders but not in non-responders. On the other hand urinary exosomal NKCC2 did not change its expression in these specific patients. *p<0.05 vs. non-responders.
Discussion
In the present study, we proved to show the efficacy of tolvaptan for CHF complicated with advanced CKD patients. It is reported that renal impairment attenuates the increase in 24 h urine volume and free water clearance upon tolvaptan treatment probably due to decreased nephron function in kidney injury [17]. To date, there are increasing numbers of reports on its efficacy for CHF patients with advanced CKD without serious adverse events [18-22], though there are a certain number of non-responders to tolvaptan. The mechanisms of tolvaptan resistance are not well understood. In the present study, about 40% of patients with CHF complicated with advanced CKD showed non-responders. Baseline data however revealed no significant difference between responders and non-responders, rendering the prediction of tolvaptan responsiveness difficult. Estimated GFR tended to be higher and urine osmolality tended to be lower in responders.
Imamura et al. demonstrated that more than 26% decrease in urine osmolality from a baseline (>352 mOsm/kgH2O) for the first 4-6 h predicts responders to tolvaptan in hospitalized CHF patients [11]. Iwatani reported that in overhydrated CKD patients with CHF or liver cirrhosis, the cut-off value of urine osmolality was 279 mOsm/kgH2O before the use of tolvaptan to predict responders to the drug [23]. In our patients, urinary osmolality showed 280 ± 52 mOsm/kgH2O in responders and 317 ± 160 mOsm/kgH2O in non-responders. Thus it appears difficult to predict responders solely by baseline urinary osmolality.
We investigated the changes in urinary exosomal AQP-2, and all of responders showed the reduction of urinary exosomal AQP-2 after treatment of tolvaptan. Excretion of AQP-2 from the renal collecting ducts into urine has been considered as a functional marker of the collecting duct [12]. Our data strongly suggest that functional vasopressin V2 receptor (V2R) and AQP-2 axis was antagonized by tolvaptan in responders, resulting in reducing urine exosomal AQP-2. In contrast, AQP-2 in nonresponders was not affected after tolvaptan treatment. It still remains unknown why there are differences of efficacy of tolvaptan among CHF patients. Tolvaptan might not effectively bind V2R by unknown reason or urinary AQP-2 does not reflect a functional marker of the collecting duct in non-responders.
Recently, Imamura and colleagues reported that responders to tolvaptan showed a close correlation of plasma arginine vasopressin (AVP) with urinary AQP-2 in patients with decompensated CHF [13]. In contrast, non-responders had extremely low urinary AQP-2 regardless of plasma AVP level, suggesting that efficacy of tolvaptan is closely associated with urinary AQP-2 level in patients with decompensated CHF. In the present study, though not quantitatively analyzed, urine AQP-2 levels before tolvaptan treatment in non-responders seemed to be comparable to that in responders. Thus, the efficacy of tolvaptan is not associated with AQP-2 level. The different results might be raised by this reason that we examined AQP-2 in urinary exosome, which may reflect more physiological condition of urinary AQP-2 excretion because the majority of AQP-2 excreted into the urine is via the exosomal pathway [24], while Imamura et al. measured AQP-2 in urine using sandwich enzyme-linked immunosorbent assay [13].
The expression of AQP-2 in the kidney tissue was reduced in a variety of kidney diseases compared to normal kidneys [25,26]. Sato reported that the efficacy of tolvaptan for treating nephrotic syndrome due to diabetic nephropathy with heart failure [27]. Responders showed mild tubulointerstitial damage along with severe diabetic glomerulosclerosis and serum creatinine of 4.0 ± 2.6 mg/dL, but non-responders exhibited profound tubulointerstitial damage and serum creatinine of 4.9 ± 3.0 mg/dL. Immunopositive AQP-2 was expressed in the epithelial cells of the collecting ducts of tolvaptan responders. These findings suggest that renal dysfunction per se dose not fully explain tolvaptan resistance. Moreover, even peritoneal dialysis patients could increase urine volume and control body fluid by tolvaptan [28,29], suggesting again that decreased renal function solely does not predict tolvaptan resistance. Degree of damages of functional V2R and AQP-2 axis in collecting ducts should contribute to tolvaptan resistance. Further study is necessary.
We found that tolvaptan did not significantly increase serum Na concentration in our patients, suggesting that tolvaptan has not only an effect of water diuresis but also an effect of sodium diuresis [30]. Urinary exosomal furosemide-sensitive NKCC2 as an indirect marker of raised renal tubular sodium transport in the thick ascending limb of Henle was not changed under taking furosemide after tolvaptan administration in both responders and non-responders to tolvaptan. Since exosome analysis is a potential approach to discover urinary biomarkers [31,32], further examination of abundance and phosphorylation sites of other sodium transporters in combination of AQP-2 in urinary exosome may be promising way to explore the mechanisms of water and/or sodium diuresis under the specific condition of interest.
The present study has several limitations. Firstly, the sample size is too small but despite the small number of the enrolled patients, the difference in response to tolvaptan is clearly shown among the advanced CKD patients. Secondly, a multivariate analysis was not feasible also due to the small size of the study. With increasing number of the patients to be included in the study, we expect to elucidate some predictor for resistance to tolvaptan, which may provide a strong clue in clinical medicine. Lastly, volume status should be evaluated in conjunction with other method such as bio impedance vector analysis (BIVA) which is an easy, fast technique to assess peripheral congestion with being even more accurate than BNP [33]. These limitations should be resolved by increasing the number of subjects and introducing sophisticated technology in the future.
In conclusions, tolvaptan resistance in CHF patients with CKD stage 3 or higher was significantly related to non-suppression of V2R and AQP-2 axis, suggesting that V2R/AQP-2 system may play a physiologically important role on over volume status in such patients. Although effectiveness of tolvaptan cannot be predicted by baseline laboratory findings, changes in urine osmolality may help foresee the drug effect within several days. Therefore, at this moment it is worth trying to introduce tolvaptan in such patients at high risk. Analysis of urinary exosome containing AQP-2 conjunction with changes in clinical parameters could elucidate pathophysiological property of tubular handling of water and sodium diuresis in CHF patients with advanced CKD.
Acknowledgments
We thank all of the doctors at the Division of Nephrology, Department of Internal Medicine, Teikyo University Hospital, Tokyo, Japan, for their cooperation.
Support
This study was supported in part by a Grant-in-Aid for Progressive Renal Diseases Research, Research on Rare and Intractable Disease, from the Ministry of Health, Labour and Welfare of Japan (to SU), Gout Research Foundation (to SU) and JSPS KAKENHI grants 16K00871 (to SU). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests
The authors have declared that no competing interests exist.
Author Contributions
Research idea and study design: SU; data acquisition: KK, EK-O, SA, MN, YT, SS; data analysis: KK, EK-O, SA, YF, SU; supervision or mentorship: SU; manuscript writing: KK, YF, SU; subject recruitment: KK, SA, MN, YT, SS, SU; interpretation of results: KK, EK-O, SA, SS, SU; reviewed the manuscript and approved the final version: KK, EK-O, SA, MN, YT, SS, YF, SU.
References
- Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S, Yokota T, Ide T, et al. (2009) Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan (JCARE-CARD). Circ J 73: 1442-1447.
- Kon V, Yared A, Ichikawa I (1985) Role of renal sympathetic nerves in mediating hypoperfusion of renal cortical microcirculation in experimental congestive heart failure and acute extracellular fluid volume depletion. J Clin Invest 76: 1913-1920.
- Wang DJ, Gottlieb SS (2008) Diuretics: still the mainstay of treatment. Crit Care Med 36: S89-94.
- Butler J, Forman DE, Abraham WT, Gottlieb S S, Loh E, et al. (2004) Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 147: 331-338.
- Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW (2000) The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol 35: 681-689.
- Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, et al. (2000) Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation 102: 203-210.
- Yamamura Y, Nakamura S, Itoh S, Hirano T, Onogawa T, et al. (1998) OPC-41061, a highly potent human vasopressin V2-receptor antagonist: pharmacological profile and aquaretic effect by single and multiple oral dosing in rats. J Pharmacol Exp Ther 287: 860-867.
- Hirano T, Yamamura Y, Nakamura S, Onogawa T, Mori T (2000) Effects of the V(2)-receptor antagonist OPC-41061 and the loop diuretic furosemide alone and in combination in rats. J Pharmacol Exp Ther 292: 288-294.
- Gheorghiade M, Konstam MA, Burnett JC, Grinfeld L, Maggioni AP, et al. (2007) Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 297: 1332-1343.
- Costello-Boerrigter L C, Smith WB, Boerrigter G, Ouyang J, Zimmer CA, et al. (2006) Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol 290: F273-278.
- Imamura T, Kinugawa K, Shiga T, Kato N, Muraoka H, et al. (2013) Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients--association between non-responders and chronic kidney disease. Circ J 77: 397-404.
- Elliot S, Goldsmith P, Knepper M, Haughey M and Olson B (1996) Urinary excretion of aquaporin-2 in humans: a potential marker of collecting duct responsiveness to vasopressin. J Am Soc Nephrol 7: 403-409.
- Imamura T, Kinugawa K, Fujino T, Inaba T, Maki H, et al. (2014) Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ J 78: 2240-2249.
- Kim GH, Ecelbarger C, Knepper MA, Packer RK (1999) Regulation of thick ascending limb ion transporter abundance in response to altered acid/base intake. J Am Soc Nephrol 10: 935-942.
- Adachi M, Asakura Y, Muroya K, Tajima T, Fujieda K, et al. (2010) Increased Na reabsorption via the Na-Cl cotransporter in autosomal recessive pseudohypoaldosteronism. Clin Exp Nephrol 14: 228-232.
- Pisitkun T, Shen R F, Knepper MA (2004) Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368-13373.
- Shoaf SE, Bricmont P, Mallikaarjun S (2014) Pharmacokinetics and pharmacodynamics of oral tolvaptan in patients with varying degrees of renal function. Kidney Int 85: 953-961.
- Imamura T, Kinugawa K, Kato N, Minatsuki S, Muraoka H, et al. (2013) Successful conversion from thiazide to tolvaptan in a patient with stage d heart failure and chronic kidney disease before heart transplantation. Int Heart J 54: 48-50.
- Otsuka T, Sakai Y, Ohno D, Murasawa T, Sato N, et al. (2013) The effects of tolvaptan on patients with severe chronic kidney disease complicated by congestive heart failure. Clin Exp Nephrol 17: 834-838.
- Matsue Y, Suzuki M, Nagahori W, Yoshida K, Onishi Y, et al. (2014) Clinical effectiveness of tolvaptan in patients with acute decompensated heart failure and renal failure: design and rationale of the AQUAMARINE study. Cardiovasc Drugs Ther 28: 73-77.
- Hirano D, Kakegawa D, Yamada A, Ito A, Miwa S, et al. (2015) Tolvaptan in a pediatric patient with diuretic-resistant heart and kidney failure. Pediatr Int 57: 183-185.
- Tominaga N, Kida K, Matsumoto N, Akashi YJ, Miyake F, et al. (2015) Safety of add-on tolvaptan in patients with furosemide-resistant congestive heart failure complicated by advanced chronic kidney disease: a sub-analysis of a pharmacokinetics/ pharmacodynamics study. Clin Nephrol 84: 29-38.
- Iwatani H, Kawabata H, Sakaguchi Y, Yamamoto R, Hamano T, et al. (2015) Urine osmolarity predicts the body weight-reduction response to tolvaptan in chronic kidney disease patients: a retrospective, observational study. Nephron 130: 8-12.
- Wen H, Frokiaer J, Kwon TH, Nielsen S (1999) Urinary excretion of aquaporin-2 in rat is mediated by a vasopressin-dependent apical pathway. J Am Soc Nephrol 10: 1416-1429.
- Kwon TH, Frokiaer J, Knepper MA, Nielsen S (1998) Reduced AQP1, -2, and -3 levels in kidneys of rats with CRF induced by surgical reduction in renal mass. Am J Physiol 275: F724-741.
- Bedford JJ, Leader JP, Walker RJ (2003) Aquaporin expression in normal human kidney and in renal disease. J Am Soc Nephrol 14: 2581-2587.
- Sato E, Nakamura T, Amaha M, Nomura M, Matsumura D, et al. (2014) Effect of tolvaptan in patients with chronic kidney disease due to diabetic nephropathy with heart failure. Int Heart J 55: 533-538.
- Mori T, Oba I, Koizumi K, Kodama M, Shimanuki M, et al. (2013) Beneficial role of tolvaptan in the control of body fluids without reductions in residual renal function in patients undergoing peritoneal dialysis. Adv Perit Dial 29: 33-37.
- Iwahori T, Esaki M, Hinoue H, Esaki S, Esaki Y (2014) Tolvaptan increases urine and ultrafiltration volume for patients with oliguria undergoing peritoneal dialysis. Clin Exp Nephrol 18: 655-661.
- Knepper MA, Kim GH, Fernandez-Llama P, Ecelbarger CA (1999) Regulation of thick ascending limb transport by vasopressin. J Am Soc Nephrol 10: 628-634.
- Zhou H, Yuen P S, Pisitkun T, Gonzales PA, Yasuda H, et al. (2006) Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int 69: 1471-1476.
- Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, et al. (2009) Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 20: 363-379.
- Massari F, Iacoviello M, Scicchitano P, Mastropasqua F, Guida P, et al. (2016) Accuracy of bioimpedance vector analysis and brain natriuretic peptide in detection of peripheral edema in acute and chronic heart failure. Heart Lung 45: 319-326.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences