The Prognostic Significance of IgG4 Deposition in Membranous Nephropathy and Its Impact on the Therapeutic Regimen
Hala Kfoury, Sufia Husain, Hisham Alkhalidi, Doaa AlGhamdi, Jose Manuel El Asmar, Patrick Bou Samra, Abdulkareem Alsuwaida, Mohammad Alkhowaiter and Mohammad Alghonaim
DOI10.21767/2472-5056.100009
Hala Kfoury1*, Sufia Husain1, Hisham Alkhalidi1, Doaa AlGhamdi2, Jose Manuel El Asmar3, Patrick Bou Samra3, Abdulkareem Alsuwaida4, Mohammad Alkhowaiter4 and Mohammad Alghonaim4
1Department of Pathology and Laboratory Medicine, College of Medicine, King Saud University, Saudi Arabia
2Department of Pathology, College of Medicine, King Abdulaziz University, Saudi Arabia
3American University of Beirut, Lebanon
4Department of Medicine, College of Medicine, King Saud University, Saudi Arabia
- *Corresponding Author:
- Hala Kfoury
Department of Pathology and Laboratory Medicine (32)
PO Box 2925 College of Medicine
King Saud University, Riyadh
11461, Saudi Arabia
E-mail: halakfoury@hotmail.com
Received date: April 12, 2016; Accepted date: May 02, 2016; Published date: May 05, 2016
Citation: Kfoury H, Husain S, Alkhalidi H, AlGhamdi D, El Asmar JM, et al. (2016) The Prognostic Significance of IgG4 Deposition in Membranous Nephropathy and Its Impact on the Therapeutic Regimen. J Clin Exp Nephrol 1:9. DOI: 10.21767/2472-5056.100009
Copyright: © 2016 Kfoury H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objectives: To assess the prognostic value of IgG4 reactivity in biopsy-proven cases of idiopathic membranous nephropathy (iMN) and to outline its potential in guiding therapy.
Methods: A retrospective study of biopsy-proven iMN cases from January 1997 to August 2013 was undertaken. Patients were identified, and an extensive analysis of the clinical and histological parameters were performed. The primary endpoint was a worse renal outcome, which was defined as doubling of the serum creatinine baseline value.
Results: The study included 52 patients, with mean age of 38.0 years. The median baseline creatinine was 112.7 ± 36.3 umol/l among those with positive staining for IgG4 and 67.4 ± 19.3 umol/l among those with negative staining for IgG4 (P=0.5). There was no significant difference in the 24 hr urine protein between IgG4 positive and IgG4 negative staining cases (P=0.375). The probability of doubling serum creatinine was similar among those with or without IgG4 deposition. Follow up of the patients revealed that 23.4% of those with positive IgG4 and 14% of those with negative IgG4 had deterioration of the renal function. The prevalence and severity of tubulo-interstitial inflammation was not statistically different between the two groups.
Conclusion: There was no relationship between IgG4 positivity and the severity of clinical and/or histological parameters in patients with iMN. IgG4 reactivity had no impact on the long-term outcome of the patients. Further studies are needed to outline the potential use of targeted therapy against IgG4 auto-antibody in a certain category of patients with membranous nephropathy.
Keywords
Membranous nephropathy; Serum creatinine; Treatment
Introduction
Membranous nephropathy (MN) is one of the most common causes of nephrotic syndrome in adults worldwide, and 75% of cases are idiopathic [1]. It is an autoimmune disease characterized by thickening of the glomerular capillary basement membrane due to inflammation [1-2]. At the molecular level, an autoimmune attack against podocytes occurs. A defect at the podocyte level hinders proper filtration and undermines kidney function by the formation of immune complexes [2-3]. The deposition of immune complexes leads to potential end-stage renal disease (ESRD). Histological findings include alterations in basement membrane structure and IgG containing subepithelial electron-dense deposits with a classical presentation of marked proteinuria and nephrotic syndrome [4].
Research studies show that neural endopeptidase antibody to neural endopeptidase of the IgG1 and IgG4 subtypes, a podocyte surface antigen is a culprit to the pathology [5]. Salant et al. proved the assembly of a membrane attack complex (C5b-C9) is a requirement for the proteinuria observed [6]. Another antibody was described by Beck in 2009 which showed the antibody against the M-type phospholipase A2 receptor (PLA2R1) present 70%-80% in the IgG4 form [7]. The presence of anti-PLA2R1 antibodies is associated with an aggressive form of the disease [8]. The anti-PLA2R antibodies involved in this disease were of the IgG4 subtype [9]. The antigen-antibody complex that is formed interfere with the podocytes’ adherence to collagen [10]. The presence of these antibodies were shown to be correlated to the clinical activity of MN [11]. Because of this, the severity of MN can be well monitored and subsequently, improve in the management of the disease [1,10].
This study was conducted to assess the prognostic value of IgG4 reactivity in a cohort of biopsy proven cases of membranous nephropathy (MN) and to outline its potential in guiding therapy.
Subjects and Methods
A retrospective study of biopsy-proven iMN cases from January 1997 to August 2013 was undertaken. A total of 87 patients with iMN were identified. Thirty-five patients were excluded due to a lack of clinical or histological data. The clinical and histological data from the remaining 52 patients were analyzed. The primary endpoint was a worse renal outcome which was defined as doubling of serum creatinine of the baseline value.
Consent has been obtained before kidney biopsy, and our study has been performed according to the Declaration of Helsinki. Ethical approval was obtained from the Institutional Review Board of King Saud University, Riyadh, Saudi Arabia.
Statistical analysis
Data are presented as frequencies, percentages, and means (SD). Frequency distribution analysis was conducted using the Statistical Package for Social Sciences (SPSS) version 18 software (IBM, SPSS Inc., Chicago, Illinois, USA).
Results
A total of 52 patients was included in the study. There were 36 (69.2%) males and 16 (30.8%) were females. The mean age was 38.0 ± 15.1 years.
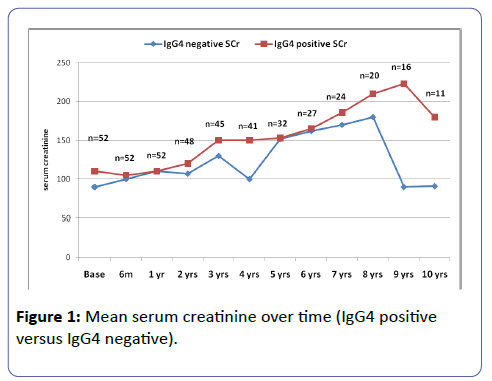
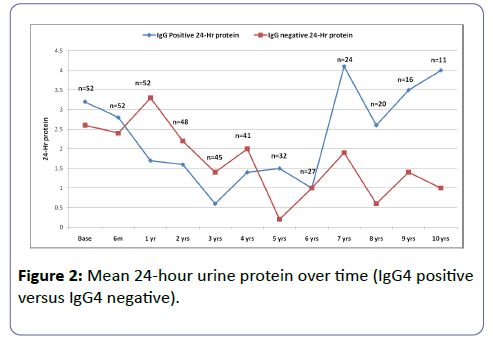
The mean follow up 5.9 years (SD 4.5 years). The median follow up was 4 years (IQR 2-9 years). Twenty-two patients (42.3%) were hypertensive, and 5 (9.6%) were diabetic. Table 1 shows the demographic characteristics of the patients. Figures 1 and 2 shows the serum creatinine and 24 hour urine protein from baseline up to 10 years of follow-up.
| n | % | ||
|---|---|---|---|
| Mean age, in years | 38.0 ± 15.1 | ||
| Gender | |||
| Males | 36 | 69.2 | |
| Females | 16 | 30.08 | |
| On dialysis | 2 | 3.8 | |
| Diabetic | 5 | 9.6 | |
| Hypertensive | 22 | 42.3 | |
| Medications | |||
| ACE inhibitor | 24 | 46.2 | |
| Angiotensin receptor blocker | 9 | 17.3 | |
| Calcium channel blocker | 8 | 15.4 | |
| Beta blocker | 10 | 19.2 | |
| Statin | 29 | 55.8 | |
| Prednisolone | 34 | 65.4 | |
| Immunosuppressive agents | 13 | 25.0 | |
| Tacrolimus | 3 | 5.8 | |
| Cyclosporine | 5 | 9.6 | |
| Mycophenolate | 2 | 3.8 | |
| Cyclophosphamide | 3 | 5.8 | |
| Mean baseline serum creatinine, umol/l | 97.3 ± 69.6 | ||
| Mean baseline 24-h urine protein, gm per day | 6.3 ± 4.4 | ||
| Mean baseline albumin, g/l | 22.3 ± 8.5 | ||
| Mean baseline cholesterol, mmol/l | 7.6 ± 3.4 | ||
| Mean baseline Hb, g/l | 131.8 ± 18.8 | ||
Table 1: Demographic characteristics of the patients (n=52).
The mean baseline creatinine was 112.7 ± 36.3 umol/l among those with positive staining for IgG4 and 67.4 ± 19.3 umol/l among those with negative staining for IgG4 (P=0.50). There was a significant difference in the median maximum creatinine between IgG4 positive and negative (119.0 ± 38.0 and 71.0 ± 19.0 respectively, P=0.034). (Table 2) There was no significant difference in the 24 hr urine protein between IgG4 positive and IgG4 negative staining cases (P=0.375). The probability of doubling serum creatinine was similar among those with or without IgG4 deposition. Follow up of the patients revealed that 23.4% of those with positive IgG4 and 14% of those with negative IgG4 had deterioration of the renal function. Of note, the number of patients who had available data after 7 years decreased to 16 than 11 only, therefore, the statistical analysis was better outlined the first 7 years. The prevalence and severity of tubulo-interstitial inflammation was not statistically different between the two groups (Table 3).
| Covariates | IgG4 ≤ 1 | IgG4 ≥ 2 | P values |
| (negative) | (positive) | ||
| Number | 10 (19.2%) | 42 (80.8%) | |
| Median follow up, Years | 4 | 4 | 0.984 |
| Male gender | 4 (40.0%) | 35 (83.3%) | 0.004 |
| Age, mean ± SD | 30.2 ± 8.4 | 39.9 ± 15.9 | 0.069 |
| Creatinine baseline, median (IQR) | 67.4 (19.3) | 102.7 (36.3) | 0.056 |
| Urine protein baseline, median (IQR), g/day | 1.24 (1.95) | 1.91 (2.65) | 0.304 |
| Creatinine max baseline, median (IQR), umol/l | 71 (19.0) | 119 (38.0) | 0.034 |
| Urine protein max baseline, median (IQR), g/day | 2.39 (2.94) | 4.12 (5.83) | 0.375 |
Table 2: Covariates between patients with negative and positive IgG4 staining.
| Variables | IgG4 ≤ 1 | IgG4 ≥ 2 | P values |
| (negative) | (positive) | ||
| Number | 10 | 42 | |
| Tubular atrophy | |||
| 0 | 2 (20.0%) | 8 (19.1%) | |
| 1 | 6 (60.0%) | 28 (66.7%) | 0.133 |
| 2 | 0 | 5 (11.9%) | |
| 3 | 2 (20.0%) | 1 (2.4%) | |
| Interstitial fibrosis | |||
| 0 | 3 (30.0%) | 8 (19.1%) | |
| 1 | 5 (50.0%) | 29 (69.1%) | 0.255 |
| 2 | 0 | 3 (7.1%) | |
| 3 | 2 (20.0%) | 2 (4.8%) | |
| GGS% (globally sclerotic glomeruli) median (IQR) | 1.22 (40.0) | 1.72 (9.52) | 0.094 |
| SGS% ( segmentally sclerotic glomeruli) mean ± SD | 2.63 ± 3.3 | 6.59 ± 6.6 | 0.256 |
| GGS, globally sclerotic glomeruli | |||
| SGS, segmentally sclerotic glomeruli | |||
Table 3:Tubular atrophy, interstitial fibrosis, GGS%, and SGS% values in patients with negative and positive IgG4 staining.
Discussion
Idiopathic membranous nephropathy is a kidney disease involving the glomerulus mostly due to the subepithelial deposition of immune complexes. The pathogenesis of iMN remains incompletely elaborated, although Beck et al. in 2009 [7], proposed the involvement of circulating autoantibodies against phospholipase A2 receptor 1 (PLA2R1). Subsequent reports on the development of iMN showed that apart from PLA2R1, there is also the involvement of human leukocyte antigen DQA1 allele that makes one susceptible to autoimmunity [12], and the production of IgG4 anti-PLA2R1, that binds to the epitopes on the surface of the podocytes to form immune complexes [10]. The dominance of IgG4 deposition is more believed to favor iMN than the secondary forms of MN, as seen in MN secondary to malignancies [13]. Furthermore, as stipulated in Xiao's study in 2013, that "an increase in the urine samples with IgG4 is indicative of membranous nephropathy among other related diseases", further affirms the role of IgG4 in MN [14]. It is believed that dysregulated IgG4 play a role in the development of iMN.
In contrast to previous reports [5-9], our study showed no relationship between the presence of IgG4 deposition and the severity of iMN. This was shown in the insignificant difference in the probability of doubling of the serum creatinine between those with positive IgG4 and those with negative IgG4. More so, the prevalence and severity of tubulo-interstitial inflammation was not statistically different between the two groups. Our results are in contrast to the published relationship between IgG4 and iMN .
The association between iMN and IgG4 in previous studies may be due to their overlapping renal manifestations as IgG4 renal disease includes iMN with or without tubulo-interstitial inflammation. Of note, the histopathological features related to the chronicity of the disease and including tubular atrophy, interstitial fibrosis segmental and global sclerosis of the glomeruli showed insignificant difference between those who have positive staining to IgG4 and IgG4 negative patients [15]. The insignificant differences is shown in Table 3.
The associations between iMN and IgG4 have report in previous studies which could be related to their overlapping renal manifestations. IgG4 renal disease and iMN can be associated with tubulo-interstitial inflammation; however, our results showed an insignificant difference between IgG4- positive and IgG4-negative patients. [15]
There are several potential explanations for our finding. The first is that, there may be an overlap in the presence of IgG4 in patients with iMN, or there may also be other unknown fundamental differences that exist between patients who show IgG4 and those who do not have IgG4. While iMN is a disease limited to the kidneys, the probability of iMN as part of multi-organ IgG4 related disease is also to be considered [16]. Furthermore, the effect of treatment, in particular, the use of glucocorticoids in our iMN patients may have reduced the levels of PLA2R and IgG4, so as to come up with an insignificant difference in the IgG4 staining positivity.
Conclusion
There was no relationship between IgG4 positivity and the severity of clinical and/or histological parameters in patients with iMN. IgG4 reactivity had no impact on long term outcome of the patients. Further studies are needed to outline the potential use of targeted therapy against IgG4 auto-antibody in a certain category of patients with membranous nephropathy.
References
- Lai WL, Yeh TH, Chen PM, Chan CK, Chiang WC, et al. (2015) Membranous nephropathy: a review on the pathogenesis, diagnosis, and treatment. J Formos Med Assoc 114: 102-111.
- Kwatra IS, Prasher PK (2013) Pathogenesis of membranous nephropathy: update. J Assoc Physicians India 61: 807-810.
- Debiec H, Ronco P (2014) Immunopathogenesis of membranous nephropathy: an update. SeminImmunopathol 36: 381-397.
- Glassock RJ (2010) The pathogenesis of idiopathic membranous nephropathy: a 50-year odyssey. Am J Kidney Dis 56: 157-167.
- Pruchno CJ, Burns MM, Schulze M, Johnson RJ, Baker PJ, et al. (1991) Urinary excretion of the C5b-9 membrane attack complex of complement is a marker of immune disease activity in autologous immune complex nephritis. Am J Pathol 138: 203–211.
- Salant DJ (2009) In search of the elusive membranous nephropathy antigen. Nephron Physiol 112: p11-12.
- Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, et al. (2009) M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11-21.
- Kanigicherla D, Gummadova J, Mckenzie EA, Roberts SA, Harris S, et al. (2013) Anti-PLA2R antibodies measured by ELISA predict Long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int 83: 940-948.
- Å koberne A, Behnert A, Teng B, Fritzler MJ, Schiffer L, et al. (2014) Serum with phospholipase A2 receptor autoantibodies interferes with podocyte adhesion to collagen. Eur J Clin Invest 44: 753-765.
- Alexander MP, Larsen CP, Gibson IW, Nasr SH, Sethi S, et al. (2013) Membranous glomerulonephritis is a manifestation of IgG4-related disease. Kidney Int 83: 455-462.
- Hofstra JM, Beck LH Jr, Beck DM, Wetzels JF, Salant DJ (2011) Anti-phospholipase A2receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am SocNephrol 6: 1286-1291.
- Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, et al. (2011) Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364: 616-626.
- Qu Z, Liu G, Li J, Wu LH, Tan Y, et al. (2012) Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol Dial Transplant 27: 1931-1937.
- Li XL, Yan TK, Li HF, Xu PC, Jia JY, et al. (2014) IgG4-related membranous nephropathy with high blood and low urine IgG4/IgG ratio: a case report and review of the literature. ClinRheumatol 33: 145-148.
- Filippone EJ (2014) Idiopathic membranous nephropathy and IgG4: an interesting relationship. ClinNephrol 82: 7-15.
- Khosroshahi A, Stone JH (2011) A clinical overview of IgG4-related systemic disease. CurrOpinRheumatol 23: 57-66.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences