Staphylococcus aureus Carriage and Bacteremia in Patients Treated with Hemodialysis: A Prospective M ulticenter Study
Rie Glerup1*, My Svensson2,6, Jens D Jensen3,7, Anders R Larsen4, Henrik C Schønheyder5,8 and Jeppe H Christensen1,8
1Department of Nephrology, Aalborg University Hospital, Aalborg, Denmark
2Department of Renal Medicine, Akershus University Hospital, Lørenskog, Norway
3Department of Renal Medicine, Aarhus University Hospital, Aarhus, Denmark
4Department of Bacteria, Parasites and Fungi, Statens Serum Institute, Copenhagen, Denmark
5Department of Clinical Microbiology, Aalborg University Hospital, Aalborg, Denmark
6Department of Nephrology, University of Oslo, Institute of Clinical Medicine, Oslo, Norway
7Department of Clinical Medicine, Aarhus University, Institute of Clinical Medicine, Aarhus, Denmark
8Department of Clinical Medicine, Aalborg University, Institute of Clinical Medicine, Aalborg, Denmark
- *Corresponding Author:
- Rie Glerup Department of Nephrology, Aalborg University Hospital, Aalborg, Denmark, E-mail: rig@rn.dk
Received date: July 05, 2022, Manuscript No. IPJCEN-22-14229; Editor assigned date: July 07, 2022, PreQC No. IPJCEN-22-14229 (PQ); Reviewed date: July 18, 2022, QC No. IPJCEN-22-14229; Revised date: July 26, 2022, Manuscript No. IPJCEN-22-14229 (R); Published date: Aug 05, 2022, DOI: 10.36648/2472-5056.7.8.151
Citation: Glerup R, Svensson M, Jensen JD, Larsen AR, Schønheyder HC, et al. (2022) Staphylococcus aureus Carriage and Bacteremia in Patients Treated with Hemodialysis: A Prospective Multicenter Study. J Clin Exp Nephrol Vol.7 No.8: 151.
Abstract
Background
Staphylococcus aureus bacteremia is frequent in hemodialysis patients. S. aureus colonization increases risk of bacteremia. Knowledge about bacterial factors increasing the risk of S. aureus bacteremia is sparse. The aims of this study were to examine S. aureus colonization and risk of bacteremia and death, and to compare strains causing bacteremia in hemodialysis patients versus other patients in the same population.
Methods
Observational, prospective multicenter study with 5-year follow-up including patients receiving chronic in-center hemodialysis in five facilities in Denmark. Baseline S. aureus nasal carriage and SABs were evaluated during follow-up. Population structures of SAB isolates from hemodialysis patients were compared to SAB isolates from the general population by comparing spa types. Time-to-event data were analyzed using Cox regression models and spa type differences using Chi-squared and Fisher exact tests.
Results
In total 336 patients were followed for 911.6 years (median 2.5 years (IQR 1.0-4.9)). Fifty patients (14.9%) experienced 72 SAB episodes. In the cohort 43% were S. aureus carriers at baseline with an increased risk of SAB compared to noncarriers (hazard ratio 2.63) and also death from all causes (hazard ratio 1.65). Hemodialysis patients experienced bacteremias with spa type’s t630, t648, t189, t474, t121 and t530 more often than the general population, a finding probably caused by within facility transfers and recurrences.
Conclusion
Hemodialysis patients colonized with S. aureus were at higher risk of SAB and all-cause death compared to noncarriers. The latter finding is novel. We did not find spa types with predilection for hemodialysis patients.
Keywords
End stage renal disease; Renal failure; Bacteraemia; Bloodstream infection; Staphylococcus aureus colonization; Spa type; Clonal Complex.
Introduction
Staphylococcus aureus is a ubiquitous bacterium colonizing both healthy and immune compromised hosts and may lead to life-threatening invasive infections if host defenses are jeopardized or skin barriers are breached [1]. S. aureus primarily colonizes the anterior nares [2,3] and prevalence studies in the general population have reported rates of colonization of 20-30% [4-7]. Colonized patients are at higher risk of developing S. aureus bacteremia and in 80% of colonized SAB cases the bacteria are of endogenous origin [5]. Case fatality in the general population due to SAB remains high and secondary infections such as endocarditis and spondylitis are frequent [8-9].
Patients treated with hemodialysis are at high risk of complicated S. aureus infections [10]. S. aureus is the most common causative pathogen in bacteremias [11] with the unadjusted Incidence Rate Ratio (IRR) being 78.3 (70.5-86.9) for SAB in HD patients compared to population controls [12]. HD patients are frequently in contact with hospitals, skin barriers are disrupted, foreign materials are inserted into the blood stream and immune function is compromised due to uremia, all factors that render the HD patient vulnerable to SAB. S. aureus nasal carrier rates are higher in HD patients than in the general population [13,14] and for HD patients dialyzed via Central Venous Catheters (CVC) the Relative Risk (RR) of SAB is 3.3 in nasal carriers compared to non-carriers [15]. Secondary S. aureus infections, such as endocarditis, prosthetic infections and osteomyelitis are found in 12-30% of SAB cases [9,16] but it seems, that mortality due to SAB in HD patients is somewhat lower than in the general population [12].
In order to determine if SAB infections are endogenous and to compare strains between different population’s molecular characterization and typing of S. aureus isolates is needed and spa typing has shown to be a usable and rapid cost-effective approach with high reproducibility and ease of use [17]. Spa typing is based on PCR amplification and sequencing of the protein A gene. The gene is conserved among S. aureus strains and is polymorphic and diverse due to deletions and duplications, making it suitable for discriminatory sequence typing. More than 19,000 different spa types have been found that can be grouped into so-called Clonal Complexes (CC), which in most cases can be deduced from the spa types using the Ridom spa server and literature searches. Only little is known about spa types and outcomes, and to our knowledge comparison of isolates causing SAB in HD patients with isolates from other patients in the adult general population nationwide has not previously been done.
The aim of this study was 1) to study S. aureus nasal carriage at baseline and the risk of subsequent SAB and all-cause death in patients from five hemodialysis units in Jutland, Denmark and 2) to evaluate if SAB isolates causing disease in HD patients differs from SAB isolates affecting the general Danish adult population by means of spa types.
Materials and Methods
Study Design
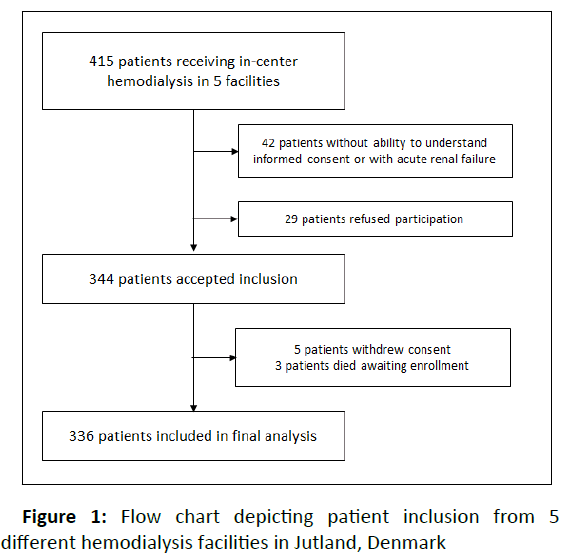
Patients were enrolled from December 2010 to March 2011 in this observational cohort study with 5 years of follow-up. Patients receiving in-center HD at five HD facilities in Jutland, Denmark (Aalborg, Aarhus, Hjorring, Randers, and Horsens), were eligible if age was >18 years, if they were on maintenance in-center HD, and they were able to understand informed consent.
Data Collection
Written informed consent was obtained from all patients. Patients underwent blood sampling, physical examination, and nasal swabbing (cotton swab placed in 6.5% saline broth). Blood samples were drawn before start of a dialysis session. Medical history was retrieved from structured patient interviews as well as medical charts at time of inclusion.
All medical records were reviewed at study entry and thrice during follow-up by one physician. Patients were followed for 5 years or censored in case of death, cessation of dialysis treatment, kidney transplantation, loss to follow-up, or change in dialysis modality to peritoneal dialysis or home HD. All S. aureus bacteremias were recorded, as were the length of hospital stay caused by SAB, bacteremia complications, focus or foci of bacteremia, and spa type of blood culture isolates. Data on bacteremias were retrieved from the Departments of Clinical Microbiology in Aarhus and Aalborg, and spa types of nasal and SAB isolates were obtained from the Danish reference laboratory (Statens Serum Institute (SSI), Copenhagen) and hospital stays as well as complications from review of medical charts. Aggregated data regarding patients with SAB in the general population was obtained from the National SAB registry at SSI and reported in the annual SAB reports (2012-2015) from SSI. The registry was established in 1957 and contains data on all SAB cases in Denmark in the period as well as typing and antimicrobial susceptibility results on all SAB isolates from each patient. Since 2010, the completeness of the voluntary submission of SAB isolates to the national surveillance has been approximately 95%. Danish resident population data used to calculate incidence rates of SAB in different age groups in the adult general population were obtained from the Danish National Bureau of Statistics (https://www.dst.dk).
Exposures and outcomes
We registered S. aureus nasal carriage at inclusion, any episode of SAB, and spa types. Blood cultures were routinely drawn in case of fever or when bacteremia was suspected for other reasons; one positive blood culture with S. aureus was sufficient to establish the diagnosis of SAB. Recurrence of SAB was regarded as a new event if more than three weeks had passed after cessation of antibiotic treatment.
Microbiology
An automated blood-culture system BacT/ALERT (BioMérieux, France) was used in North Denmark Region (Aalborg and Hjorring) and the Bactec 9240 system (Becton Dickinson, Cockeysville, MD, USA) in the Central Denmark Region (Aarhus, Randers and Horsens).
Each nasal swab was placed in 6.5% NaCl broth and incubated overnight at 37°C. Culture of S. aureus was done on 5% blood agar plates (SSI Diagnostica, Denmark) in CO2, and identification by characteristic green colonies on S. aureus ID agar (SAID; ChromID S. aureus agar, BioMérieux, France) and a rapid latex agglutination test (Monostaph Plus, Bionor, Norway). Antimicrobial susceptibility testing to detect methicillin resistance was done for cefoxitin on Muller Hinton agar plates (E and O Laboratories Ltd., Scotland).
S. aureus isolates from nasal swabs and positive blood cultures were referred to SSI for spa typing [18], spa types were determined using Bionumerics 6.1 or 6.6 (Applied Maths, Sint- Martens-Latem, Belgium) and RidomStaphType 1.4 (Ridom GmbH, Würzburg, Germany). Finally, the annotated spa types were approximated to Multi Locus Sequence Typing (MLST) CCs using the spa server homepage (https://spa.ridom.de/ spatypes.shtml), literature searches, the MLST homepage and eBURST (http://saureus.mlst.net/).
Statistical Analyses
Baseline data are given as means ± standard deviation, medians with interquartile range or percentages, as appropriate.
Age group comparisons with the adult general Danish population was done by calculating the sum of all adults in age groups of 10 years from the catchment areas of the Departments of Clinical Microbiology delivering SAB data to SSI during the years 2011-2015 (Supplemental text 1).
Prevalence of spa types and CCs is given as percentages of all bacteremia isolates submitted to the voluntary national surveillance of SAB at SSI. Difference in risk of bacteremia with specific spa type/CC compared to the rest of the Danish population is given as risk ratios with 95% Confidence Interval (CI) and p-values calculated using Chi-square test or Fisher exact test when samples were less than 5.
Survival analysis comparing S. aureus nasal carriers to noncarriers was performed using univariable and multivariable Cox regression analyses censoring patients experiencing competing events. For time-to-event analysis of SAB risk, patients were censored at the time of an event disregarding follow-up time hereafter. Multivariable survival analysis for all-cause death was adjusted for age, sex, total time on renal replacement therapy, body weight, diabetes, smoking habits, history of cardiovascular disease, and access type/cannulation technique (reference CVC compared to stepladder cannulation technique used in an arteriovenous fistula or arteriovenous graft or buttonhole cannulation in an AVF).
Statistical analyses were performed with STATA (Version 14 MP, Stat Corp, TX, US) and the level of statistical significance was set at 5%.
Ethics
The study protocol was created in accordance with the ethical principles of the Declaration of Helsinki and approved by the Regional Research Ethics Committee of The North Jutland Region (protocol N-20100041).
Results
Study Participants
Of 415 patients receiving in-center HD in the five HD facilities, 336 were included in the study (Figure 1). These patients were followed for a median of 2.5 years (IQR 1.0-4.9) and a total of 911.5 years (453 years with buttonhole cannulated AVFs, 360 years with area/stepladder cannulated AVF, 29.6 years with AVG, and 68.8 years with CVC). The median age was 67 years and 64% were males (Table 1). Forty-three percent were S. aureus nasal carriers at baseline. Active smoking and a history of cardiovascular disease were more prevalent in non-nasalcarriers of S. aureus. Otherwise, nasal carriers and non-carriers had similar baseline characteristics.
| Characteristics | Baseline carrier | Swab-negative at baseline N=193 | All patients N=336 |
|---|---|---|---|
| N=143 | |||
| Age, years | 66.3 (52.4-76.7) | 67.1 (58.5-76.0) |
67.0 (56.5-76.5) |
| Age >65 years, N (%) | 74 (52) | 109 (56) | 183 (54) |
| Male gender, N (%) | 92 (64) | 123 (64) | 215 (64) |
| Diabetes: | |||
| No, N (%) | 106 (74) | 139 (72) | 245 (73) |
| Type 1, N (%) | 14 (10) | 9 (5) | 23 (6.8) |
| Type 2, N (%) | 23 (16) | 45 (23) | 68 (20) |
| Tobacco use: | |||
| Never, N | 57 (40) | 53 (27) | 110 (33) |
| Previous, N | 59 (41) | 78 (40) | 137 (41) |
| Active, N | 27 (19) | 62 (32) | 89 (26) |
| Etiology of ESRD | |||
| Diabetic nephropathy, N (%) | 24 (17) | 38 (20) | 62 (18) |
| Glomerulonephritis, N (%) | 22 (15) | 19 (9.8) | 41 (12) |
| Vasculitis, N (%) | 6 (4.2) | 8 (4.1) | 14 (4.2) |
| Hypertension and vascular, N (%) | 18 (13) | 28 (15) | 46 (14) |
| Polycystic kidney disease, N (%) | 13 (9.1) | 19 (9.8) | 32 (9.5) |
| Uropathy, N (%) | 8 (5.6) | 15 (7.8) | 23 (6.8) |
| Other, N (%) | 22 (15) | 30 (16) | 52 (15) |
| Unknown, N (%) | 30 (21) | 36 (19) | 66 (20) |
| Previous cardiovascular disease, N (%) | 62 (43) | 107 (54) | 169 (50) |
| TTRRT, years | 3.4 (1.5-10.3) | 3.4 (1.4-7.1) | 3.4 (1.4-8.1) |
| HD vintage, years | 2.5 (1.0-6.1) | 2.4 (0.9-5.0) | 2.5 (1.0-5.3) |
| Vascular access: | |||
| AVF, N (%) | 119 (83) | 167 (87) | 286 (85) |
| AVG, N (%) | 4 (2.8) | 4 (2.1) | 8 (2.4) |
| CVC, N (%) | 20 (14) | 22 (11) | 42 (13) |
| Self-cannulation, N (%) | 11 (7.7) | 13 (6.7) | 24 (7.1) |
| Access vintage, years | 2.3 (0.9-6.2) | 2.0 (0.9-4.4) | 2.1 (0.9-4.9) |
| HD frequency, sessions/week | |||
| <3, N (%) | 28 (20) | 32 (17) | 60 (18) |
| 3, N (%) | 102 (71) | 147 (76) | 249 (74) |
| >3, N (%) | 13 (9.1) | 14 (7.3) | 27 (8.0) |
| Statin treatment, N (%) | 45 (31) | 74 (38) | 119 (35) |
| Erythropoietin treatment, N (%) | 127 (89) | 179 (93) | 306 (91) |
| Iron treatment, N (%) | 111 (78) | 145 (75) | 256 (76) |
| Immunosuppressive treatment, N (%) | 15 (10) | 13 (6.7) | 28 (8.3) |
| Immunosuppressive last 6 months N (%) | 4 (2.8) | 5 (2.6) | 9 (2.7) |
| Active cancer at inclusion, N (%)a | 8 (5.6) | 14 (7.3) | 22 (6.5) |
| Previous cancer, N (%)a | 11 (7.7) | 27 (14) | 38 (11) |
| C-reactive protein, mg/l | 6.3 (2.2-14.2) | 5.4 (2.4-15.4) | 5.8 (2.3-15.0) |
| Antibiotics at inclusion, N (%) | 8 (5.6) | 19 (10) | 27 (8.0) |
| Antibiotics last month, N (%) | 17 (12) | 32 (17) | 49 (15) |
| Subjective sign of infection, N (%) | 23 (16) | 35 (18) | 58 (17) |
| Objective signs of infection, N (%) | 11 (7.7) | 22 (11) | 33 (10) |
| Positive blood culture, all, N (%) | 6 (4.2) | 9 (4.7) | 15 (4.5) |
| Positive blood culture from peripheral vein, N (%) | 0 (0) | 2 (1.0) | 2 (0.6) |
| Blood culture with CoNS, N (%) | 5 (3.5) | 8 (4.1) | 13 (3.9) |
| Blood culture with Staphylococcus aureus, N (%) | 1 (0.7) | 1 (0.5) | 2 (0.6) |
Table 1: Baseline demographic characteristics of hemodialysis patients by Staphylococcus aureus carrier status at baseline
Data are presented as means ± SD, medians (range of 25th to 75th percentiles) or percentages where appropriate. ESRD- End Stage Renal Disease; TTRRT- Total Time on Renal Replacement Therapy; HD-Hemodialysis; AVF-Arteriovenous Fistula; AVGArteriovenous Graft; CVC-Central Venous Catheter; CoNSCoagulase Negative Staphylococci; except basal cell carcinoma and squamous cell carcinoma of the skin.
S. aureus bacteremia
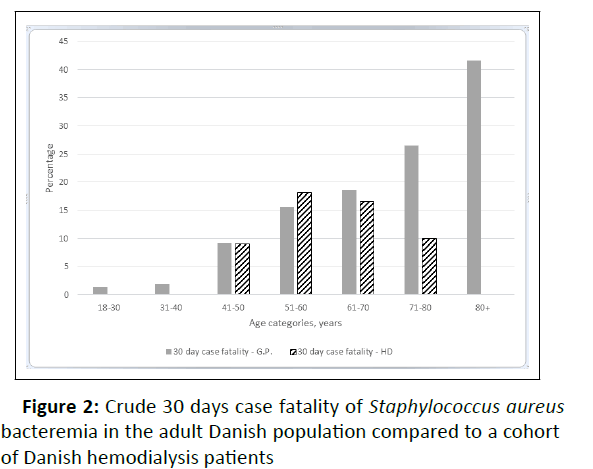
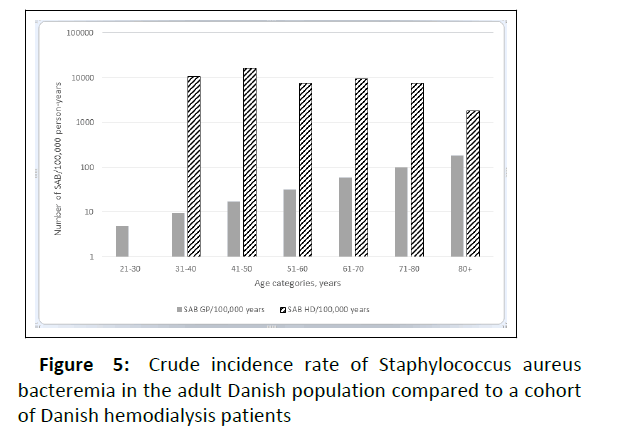
During the entire ofllow-up, 50 patients ex perienced 72 episodes of SAB, all methicillin susceptible S. aureus isolates. Twenty-five patients had 29 SAB episodes within the first year of follow-up. A single episode of SAB was observed in 38 patients, whereas eight patients experienced two episodes of SAB, and four patients had three or more episodes yielding a total Incidence Rate (IR) of 79.0 per 1,000 Person-Years of Follow-Up (PYFU) (IR of first SAB: 54.9 per 1,000 PYFU). More men than women had SAB (75% men vs. 25% women – and the Incidence Rate Ratio (IRR) was 1.90 (95% CI 1.01 – 3.58) for men compared to women). At time of infection 14 patients were dialyzed using a CVC, nine were using stepladder/area cannulation technique (eight in AVFs and one in an AVG), and 49 were using buttonhole cannulation technique. The median age at first SAB was 67.9 years (IQR: 60.4-73.6). In contrast to the general adult Danish population where SAB incidence rates increased across age groups we observed a comparable rate in the age groups, except for a decline in the age group 80+ (Figures 2 and 3).
Characterization of SAB strains and comparison with the general population
Sixty-nine isolates from 47 patients were available for spa typing; one isolate was spa PCR-negative. A total of 45 different spa types were identified, of which 32 spa types were observed only once. The 13 remaining spa types comprised 52% of all isolates, with t630 (n=5), t230 (n=4) and t648 (n=4) being the predominant ones (Table 2). Compared to the adult Danish population from 2011-2015 we observed a higher prevalence of spa type’s t630, t648 and t189 along with three other types observed in very small numbers (Table 2). The prominence of t630 and t648 were mainly caused by recurrences in one HD patient. The three patients experiencing SAB with spa type t189 were dialyzed in the same HD facility and were non-carriers at baseline. These SABs occurred 7 and 11 months apart. Two SAB cases involving t474 in two patients occurred within one facility as well – 14 months apart.
| spa type | HD cohort | Adult general population | Risk ratio |
|---|---|---|---|
| 2011-2015 (69 samples) | SSI 2011-2015 (8230 samples) | (95% CI) | |
| N (%) | N (%) | ||
| t630 | 5 (7.2) | 23 (0.3) | 25.9 (10.2-66.2)a |
| t230 | 4 (5.8) | 419 (5.1) | 1.1 (0.44-3.0) |
| t648 | 4 (5.8) | 8 (0.1) | 59.6 (18.4-193)a |
| t189 | 3 (4.3) | 73 (0.9) | 4.9 (1.6-15.2)b |
| t021 | 3 (4.3) | 180 (2.2) | 2.0 (0.65-6.1) |
| t091 | 3 (4.3) | 223 (2.7) | 1.6 (0.53-4.9) |
| t002 | 2 (2.9) | 329 (4.0) | 0.73 (0.18-2.9) |
| t008 | 2 (2.9) | 189 (2.3) | 1.3 (0.32-5.0) |
| t015 | 2 (2.9) | 238 (2.9) | 1.0 (0.25-3.9) |
| t346 | 2 (2.9) | 79 (1.0) | 3.0 (0.76-12.0) |
| t474 | 2 (2.9) | 1 (0.01) | 252 (23-2744)a |
| t121 | 2 (2.9) | 5 (0.06) | 42 (8.6-204) b |
| t530 | 2 (2.9) | 2 (0.02) | 126 (18-881)a |
Table 2: Predominant spa types among Staphylococcus aureus bacteremia isolates from hemodialysis patients in five facilities in Jutland – compared to Staphylococcus aureus bacteremia isolates from Danish adults
HD-Hemodialysis; SAB-Staphylococcus aureus Bacteremia; SSIStatens Serum Institute (the Danish Reference laboratory for Staphylococcus aureus) – excluding isolates from our cohort; pts, patients; ap <0.001; bp<0.05
Putative assignment to MLST CC was possible for 62 isolates (90%). In the remaining 10%, assignment was not possible due to a low number of repeats in the spa type (<5) or an unresolved relationship with MLST typing [13-17].
A total of 11 CC groups were assigned. The three most prevalent groups CC45, CC30 and CC8 accounted for 54% of the SAB isolates (Table 3). Compared to isolates from SAB in the adult general Danish population from 2011-2015 we found a slightly higher frequency of CC8 (RR 2.1(95% CI 1.19-3.77)) in our cohort, mainly caused by recurrences in one patient. All other CCs were distributed similarly in our cohort of HD patients and the general population.
| Clonal complex | HD cohort | Adult general population | Relative risk | Number of different spa-types (most prevalent type(s)) |
|---|---|---|---|---|
| 2011-2015 | SSI 2011-2015 (8230 samples) | (95% CI) | ||
| (69 samples) | N (%) | |||
| N (%) | ||||
| CC45 | 17 (24.6) | 1496 (18.2) | 1.36 (0.89-2.1) | 9 (t630, t230) |
| CC30 | 10 (14.5) | 1079 (13.1) | 1.11 (0.62-2.0) | 8 (t021, t021) |
| CC8 | 10 (14.5) | 571 (6.9) | 2.09 (1.2-3.7)b | 4 (t648) |
| CC15 | 7 (10.1) | 890 (10.8) | 0.98 (0.46-1.9) | 6 (t346) |
| CC22 | 5 (7.2) | 262 (3.2) | 2.3 (0.97-5.3) | 4 (t474) |
| CC1 | 4 (5.8) | 655 (8.0) | 0.73 (0.28-1.9) | 2 (t189) |
| CC5 | 3 (4.3) | 666 (8.1) | 0.54 (0.18-1.6) | 2 (t002) |
| CC7 | 3 (4.3) | 270 (3.3) | 1.3 (0.44-4.0) | 1 (t091) |
| Othersa | 3 (4.3) | 1123 (13.6) | - | 3 |
| unknown | 6 (7.2) | 1145 (13.9) | - | 6 |
| Negative PCR | 1 (2.9) | 73 (0.9) | - | N/A |
Table 3: Clonal Complexes among Staphylococcus aureus bacteremia isolates from hemodialysis patients in five facilities in Jutland – compared to Staphylococcus aureus bacteremia isolates from Danish adults
SSI-Statens Serum Institute (the Danish Reference laboratory for Staphylococcus aureus); CI-Confidence Interval; PCRPolymerase Chain Reaction aCC59, CC121, CC151; bp<0.05
S. aureus colonization
At baseline 143 patients (43%) were S. aureus nasal carriers – one patient with two strains. A total of 80 different spa types were found, with 13 of the most prevalent types comprising 42% of all isolates (Supplemental Table 1) and 55 types were found only once. Twenty-one percent of baseline carriers developed SAB during follow-up (60% of first time SAB) compared to 10% of non-carriers (hazard ratio (HR) 2.63 (95% CI 1.49-4.64) for a baseline carrier compared to a non-carrier). Nineteen cases of SAB (26%) were caused by an isolate with a spa type identical to the nasal isolate cultured at baseline and were considered endogenous. These SAB occurred within a range from 60 to 1305 days after inclusion. Baseline carriers of spa type’s t091, t094, t171, t223 and t5475 all developed SAB with the same spa type during follow up (Table 4) and for comparison none of seven carriers of t084 developed endogenous SAB. Adding to the amount of possible endogenous SAB, 8 of the total 72 events were recurrences with the same strain as the prior event. Smoking was less frequently associated with S. aureus colonization. Evaluating whether this reduced carrier rate had an impact on SAB risk yielded a HR for SAB of 2.5 (95% CI 1.1-5.6) for a never-smoker compared to an active smoker in an age and sex adjusted model. However controlling for S. aureus carrier status in the Cox model rendered the result non-significant.
| spa type | Nasal carriage at baseline | Later SAB with same spa type (endogenous) N | Clonal complex |
|---|---|---|---|
| N | |||
| t002 | 9 | 1 | CC5 |
| t084 | 7 | 0 | CC15 |
| t230 | 6 | 3 | CC45 |
| t015 | 6 | 2 | CC45 |
| t012 | 5 | 1 | CC30 |
| t127 | 4 | 0 | CC1 |
| t026 | 4 | 0 | CC45 |
| t216 | 4 | 0 | CC59 |
| t1494 | 4 | 0 | CC15 |
| t008 | 3 | 1 | CC8 |
| t021 | 3 | 2 | CC30 |
| t375 | 3 | 0 | CC509 |
| t701 | 3 | 0 | CC8 |
Supplemental Table 1: Predominant spa types among S. aureus nasal carriage isolates from patients treated with hemodialysis in five facilities in Jutland, Denmark.
| spa type | Clonal complex | Nasal carriers at baseline, N | Nasal carriers at baseline with later SAB with same spa type, N |
|---|---|---|---|
| t230 | CC45 | 6 | 3 |
| t091 | CC7 | 2 | 2 |
| t015 | CC45 | 6 | 2 |
| t021 | CC30 | 3 | 1 |
| t002 | CC5 | 9 | 1 |
| t008 | CC8 | 3 | 1 |
| t012 | CC30 | 5 | 1 |
| t089 | CC30 | 2 | 1 |
| t094 | CC15 | 1 | 1 |
| t171 | CC121 | 1 | 1 |
| t223 | CC22 | 1 | 1 |
| t550 | CC45 | 2 | 1 |
| t5475 | Unknown | 1 | 1 |
Table 4: spa types in baseline nasal swabs causing Staphylococcus aureus bacteremia in patients treated with hemodialysis in five facilities in Jutland, Denmark.
SAB- Staphylococcus aureus Bacteremia
Secondary SAB infections
Thirteen patients experienced 14 cases of secondary SAB infections (endocarditis (n=11), spondylitis (n=3), septic arthritis (n=1), and CNS infections (n=2)) – hence 15% of SAB episodes led to endocarditis. In comparison 11% of episodes in the general population led to endocarditis [13-17]. Of 13 patients experiencing a secondary SAB infection, only one was an active smoker. The isolates causing secondary SAB infections were of 14 different spa types and 12 different CCs (Supplementary Table 2). In three cases the patients were nasal carriers at baseline of the same spa type that later caused secondary SAB infection.
| spa type | Clonal complex | Same spatype at baseline? | Type of infection |
|---|---|---|---|
| t002 (1/2) | CC5 | no | Spondylitis |
| t005 (1/1) | CC22 | no | Endocarditis + spondylitis + encephalitis |
| t015 (1/2) | CC45 | yes | Spondylitis |
| t171 (1/1) | CC121 | yes | Endocarditis |
| t189 (1/3) | CC1 | no | Endocarditis |
| t223 (1/1) | CC22 | yes | Endocarditis |
| t238 (1/1) | CC30 | no | Endocarditis |
| t279 (1/1) | CC15 | no | Arthritis |
| t487 (1/1) | CC45 | no | Endocarditis |
| t529 (1/1) | CC151 | no | Endocarditis + meningitis |
| t530 (1/2) | CC8 | no | Endocarditis |
| t3599 (1/1) | CC59 | no | Endocarditis |
| t9392 (1/1) | Unknown | no | Endocarditis |
| unable to type | unable to type | N/A | Endocarditis |
Supplemental Table 2: spa types causing secondary infection among S. aureus bacteremia isolates from patients treated with hemodialysis in five facilities in Jutland, Denmark.
Mortality
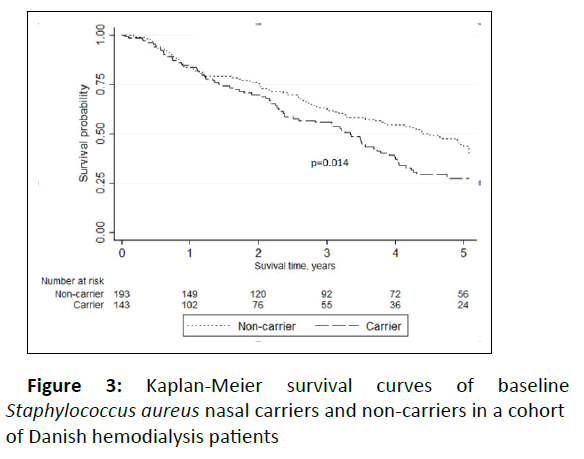
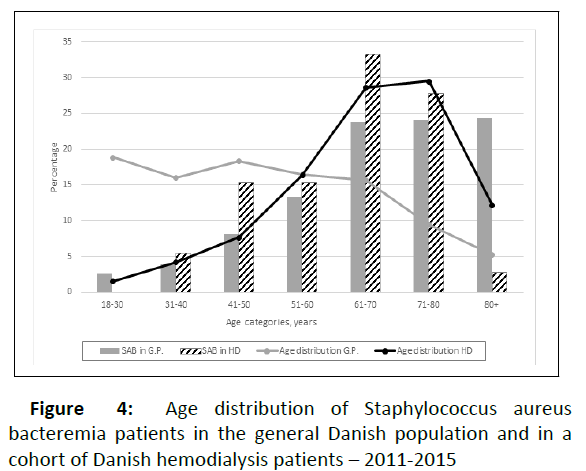
A total of 200 patients died, 31 of these because of cessation of HD treatment due to severe multimorbid conditions. Of the remaining 169 deaths during follow-up 43 (25%) were caused by infection. Nine patients died within 30 days of a SAB (12.5%), and these SABs were caused by S. aureus isolates with nine different spa types. Of interest, four of these SAB (44%) were caused by CC 45 and only one of the nine was found in nasal carriage at baseline. Three of nine case fatalities were in patients who were nasal carriers of different strains at baseline. For comparison, the 30-day case fatality from SAB in the Danish adult population was 24.0% in the years 2011-2015. The case fatality rate in our cohort was comparable with the general population in all age groups up to 70 years, with declining rates in older HD patients whilst increasing in the general population (Figure 4). Looking at all-cause mortality, baseline nasal carriers had a significantly increased risk of death with a HR of 1.46 (95% CI 1.08-1.98) in univariable analysis compared to non-carriers (Figure 5). Adjusting for several possible confounders in multivariable Cox regression analysis yielded a HR of all-cause death for carriers of 1.65 (95% CI 1.20-2.27) compared to noncarriers with increasing age, male sex, previous cardiovascular disease, and presence of a CVC being positively associated with mortality.
Discussion
This prospective multicenter study is to our knowledge the first to compare S. aureus isolates causing bacteremia in HD patients with isolates causing SAB in the adult general Danish population by means of spa types and clonal complexes. Furthermore, we report significantly increased risk of death in nasal carriers of S. aureus compared to non-carriers and confirm prior findings of an increased risk of SAB in carriers.
In line with previous studies [6,7,19] we found the nasal carrier rate to be lower in active smokers compared to nonsmokers. A possible explanation for this might be the toxicity of smoke and its ability to inhibit the growth of gram-positive bacteria [20]. In contrast to a study reporting an increased risk of bacteremia in active smokers [21] we found that smoking might reduce the risk of SAB, a finding that was not significant when adjusting for S. aureus nasal carrier state. Even though a recent in vitro study [22] found cigarette smoke to alter the virulence profile of S. aureus and causing the isolates to become associated with persistent infection we did not find SAB episodes in smokers to be more severe than in non-smokers.
The IR of first SAB in HD patients was high (54.9 per 1,000 PYFU), which corresponds well to previous Danish nationwide studies of HD patients. Nielsen et al.15 found an overall IR of 46.3 per 1,000 PYFU from 1992-2009 including all hemodialysis patients in Denmark. Another study of a similar cohort [23] found the IR to increase over the study period (1996-2011).
In contrast to the general Danish population where the IR of SAB continues to increase with increasing age, our results were not affected by age. This finding might partly be explained by the distribution of cannulation techniques across age groups in our cohort where buttonhole users were younger than stepladder users. We have recently showed, that buttonhole cannulation technique increases the risk of SAB more than 6 fold compared to stepladder/area cannulation [24].
The spa type t189 caused SAB more often in our cohort of dialysis patients compared to the general population. All cases of t189 SAB occurred in one HD facility during a 1.5 years period. Spa t474 was also more common among HD patients, but again this finding was restricted to a single HD unit. Type’s t630 and t648 were in the top three of SAB-causing spa types in our cohort, but this finding was driven by multiple recurrences in one patient. Thus, we found statistically significant differences in the spa types causing SAB in our HD patients compared to the Danish population, but these differences are probably caused by in-facility transmissions and recurrences rather than specific strain characteristics that gives the isolate greater opportunity to cause SAB in HD patients.
In a study comparing 100 SABs in HD patients with 100 SABs in post-surgical patients in the US [25] CC8 was most prominent in HD patients and CC30 in surgical patients. Our data are in accordance with the high prevalence of CC8 among HD patient, but the finding was mainly driven by recurrences in one patient. In contrast to our study McNiclolas et al. [26] found in a small study that CC45 was less prevalent among Irish HD patients compared to non-HD patients.
Our baseline S. aureus carrier rate of 43% is in line with recent reports [13, 14]. Young et al. [2] showed that at single nose swab in healthy individuals detected the majority of carriers, and that 88% of positive patients when swabbed once were persistent carriers. In 86 German HD patients Scheuch et al. [14] recently showed that an average of 40% of HD patients were carriers in cross-sectional analyses and 65% were carriers longitudinally - only 6% were carriers of the same strain of S. aureus throughout the sampling period of 25 months. Nineteen of our patients developed SAB up to 3.5 years after inclusion with an isolate of the same spa type as found in nasal swab at baseline, indicating that most of the persistent carriers developed endogenous SAB. Verhoeven et al. [27] found that HD patients that were persistent carriers of S. aureus had an increased risk of endogenous S. aureus infection compared to non-persistent carriers, which confirmed previous data in peritoneal dialysis patients [28].
In contrast to some previous studies [16, 29] we did not find associations between CC of SAB isolates and risk of secondary infections. Neither did Lilje et al. [30] find any genetic associations in SAB isolates to infective endocarditis using whole genome sequencing in 241 samples, suggesting that any opportunistic pathogen given the optimal conditions in the host may have the ability to cause endocarditis from bacteremia.
The 30-day case fatality after SAB of 12.5% in the present study was low compared to the general Danish population. A recent study [31] found SAB with CC8 and CC5 to be associated with higher mortality in a large cohort including 12% dialysis patients. Patients in our cohort were not infected less frequently with these CCs than the general population. We have too few case fatalities to exclude the presence of a strain related mortality risk, but the lower mortality observed among HD patients with SAB compared to the general population is probably due to early diagnosis, early initiation of correct therapy, and fewer cases in the older more frail age groups rather than to spa types and clonal complexes. The low rate of Methicillin Resistant S. aureus in blood isolates in Denmark might also play a role in an overall favorable outcome of SAB (ref Cassini 2019). In our cohort no cases of MRSA bacteremia was observed. In the general population, the rates of SAB caused by MRSA are also low at 1.9% of all isolates in the years 2011-2015 combined (ref 2011-2015), and despite an increase in detected MRSA-isolates over recent years, specially live-stock associated, (ref MRSA 2018) the rate of invasive infection remains relatively constant (1.6% in 2018)(ref MRSA 2018).
All-cause mortality in the present study was higher in carriers than in non-carriers, also in adjusted analysis. This finding is in contrast to other studies, that found either no difference [32,33] or a higher mortality in non-carriers [14]. However, these three studies were smaller in sample size and/or shorter in duration of follow-up compared to our study, which might explain the discrepancy. Whether eradication of carrier state abolishes the increased risk of death remains to be elucidated. Price et al. [34] did not find difference in all-cause mortality risk in HD patients that were successfully eradicated compared to patients where eradication failed.
Limitations: A limitation to our study is the lack of repeat nasal swabs for S. aureus nasal carriage to distinguish between persistent and transient carriers. Extra-nasal, e.g. pharyngeal carriage e.g. pharyngeal does occur, and sampling from these sites might have increased the detection rate [2]. Without whole genome sequencing we cannot be certain, that two isolates of the same spa type found in a patient, is in fact similar. Furthermore, in our approach for preparation of figure 2, 3, we might have overestimated slightly the size of the population and potentially underestimated the SAB incidence (Supplemental text 1). At SSI two identical isolates drawn one month apart from the same patient were regarded as two separate SAB events, even though the patient still may be receiving treatment for secondary infections (e.g. endocarditis) – hence potentially overestimating the true incidence of SAB in the general population.
SAB-Staphylococcus aureus Bacteremia; G.P-General Population; HD-Hemodialysis
Y-axis on logarithmic scale. SAB-Staphylococcus aureus Bacteremia; G.P-General Population; HD-Hemodialysis
In conclusion, we found that 43% of HD patients in a large multicenter cohort were S. aureus nasal carriers at baseline. We found that not only did these carriers have an expected increased risk of SAB; they also showed significant increased risk of all-cause death in multivariable analysis. Compared to the general population, we found a significantly different distribution of S. aureus spa types from blood isolates in our cohort, but these differences are probably due to recurrences and facility transfers rather than specific strain characteristics that facilitate easier access to the circulation of hemodialysis patients.
Supplemental material:
Supplemental Table 1: Predominant spa types among S. aureus nasal carriage isolates from patients treated with hemodialysis in five facilities in Jutland, Denmark.
Supplemental Table 2: Spa types causing secondary infection among S. aureus bacteremia isolates from patients treated with hemodialysis in five facilities in Jutland, Denmark.
Supplemental Text 1: Calculation of size of reference population divided into age groups
The size of the reference population was calculated using data from the Danish National Bureau of Statistics ( https:// www.dst.dk/). Strains causing SAB in HD patients were compared to strains causing SAB in the general adult Danish population. The adult general population was in this case defined as persons >18 years residing in the catchment areas of the Departments of Clinical Microbiology (DCM) delivering SAB data to SSI during the years 2011-2015.
For each year the population size was evaluated at the 1st of January, disregarding any deaths or emigrations. The population was divided into age categories (18-30, 31-40, 41-50, 51-60, 61-70, 71-80 and 80+) based on a residents age on the 1st of January in a given year. All Danish DCMs but one delivered data to SSI during the years 2011-2015. In 2011-2013 plus the first six months of 2014 the DCM in Sonderborg, did not submit SAB isolates, hence the reference population was comprised of all Danish adults with residents from the counties of Aabenraa, Sonderborg, Tonder and Haderslev deducted in the first 3.5 years.
Article Information
Authors’ Contributions
Research idea and study design: RG, MS, HCS, JHC; Data acquisition and statistical analyses: RG; Data interpretation: All authors. Each author contributed important intellectual content during manuscript drafting and revision.
Support
This work was supported by:
The Danish Kidney Society (DKS), The Danish Society of Nephrology (DSN), The North Denmark Region (NDR), Karen Elise Jensen's Foundation (KEJF), The Hertha Christensen Foundation (HCF), The Helen and Ejnar Bjørnow Foundation (HEBF), medical specialist Heinrich Kopp's Grant, Lundbeck Foundation (LF), The Spar Nord Foundation (TSNF) and The Obel Family Foundation (TOFF).
The funders had no role in study design; Collection, analysis, and interpretation of data; Writing the report; or the decision to submit the report for publication.
Acknowledgements
We thank the patients and staff of the HD-units participating in this study. Isolates from SAB cases were received from all Danish Departments of Clinical Microbiology. We are grateful for their voluntary submission and thank Andreas Petersen from Department of Bacteria, Parasites and Fungi, Statens Serum Institute (SSI), Copenhagen, Denmark for retrieving data from the National SAB registry.
Financial Disclosure
The authors declare that they have no relevant financial interests.
References
- Wertheim HF, Melles DC, Vos MC, Leeuwen WV, Belkum AV, et al. (2005) The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 5: 751-762.
[Crossref], [Googlescholar], [Indexed]
- Young BC, Votintseva AA, Foster D, Godwin H, Miller RR, et al. (2017) Multi-site and nasal swabbing for carriage of Staphylococcus aureus: what does a single nose swab predict? J Hosp Infect 96: 232-237.
[Crossref], [Googlescholar], [Indexed]
- Mulcahy ME, McLoughlin RM. (2016) Host-Bacterial Crosstalk Determines Staphylococcus aureus Nasal Colonization. Trends Microbiol 24: 872-886.
[Crossref], [Googlescholar], [Indexed]
- den Heijer CD, van Bijnen EM, Paget WJ, Pringle M, Goossens H, et al. (2013) Prevalence and resistance of commensal Staphylococcus aureus, including meticillin-resistant S aureus, in nine European countries: a cross-sectional study. Lancet Infect Dis 13: 409-415.
[Crossref], [Googlescholar], [Indexed]
- Wertheim HF, Vos MC, Ott A, Belkum AV, Voss A, et al. (2004) Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364: 703-705.
[Crossref], [Googlescholar], [Indexed]
- Andersen PS, Larsen LA, Fowler VG, Stegger M, Christensen K. (2013) Risk factors for Staphylococcus aureus nasal colonization in Danish middle-aged and elderly twins. Eur J Clin Microbiol Infect Dis 32: 1321-1326.
[Crossref], [Googlescholar], [Indexed]
- Olsen K, Falch BM, Danielsen K, Johannessen M, Ericson Sollide JU, t al. (2012) Staphylococcus aureus nasal carriage is associated with serum 25-hydroxyvitamin D levels, gender and smoking status. The Tromso Staph and Skin Study. Eur J Clin Microbiol Infect Dis 31: 465-473.
[Crossref], [Googlescholar], [Indexed]
- Ammerlaan H, Seifert H, Harbarth S, Brun-Buisson C, Torres A, et al. (2009) Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis 49: 997-1005.
[Crossref], [Googlescholar], [Indexed]
- Chang FY, MacDonald BB, Peacock JE, Musher DM, Triplett P,et al. (2003) A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 82: 322-332.
[Crossref], [Googlescholar], [Indexed]
- Fitzgerald SF, O'Gorman J, Morris-Downes MM, Crowley RK, Donlon S, et al. (2011) A 12-year review of Staphylococcus aureus bloodstream infections in haemodialysis patients: more work to be done. J Hosp Infect 79: 218-221.
[Crossref], [Googlescholar], [Indexed]
- Skov Dalgaard L, Norgaard M, Jespersen B, Jensen-Fangel S, Jørgen Østergaard L, et al. (2015) Risk and Prognosis of Bloodstream Infections among Patients on Chronic Hemodialysis: A Population-Based Cohort Study. PLoS One 10: p0124547.
[Crossref], [Googlescholar], [Indexed]
- Nielsen LH, Jensen-Fangel S, Benfield T, Skov R , JespersenB, et al. (2015) Risk and prognosis of Staphylococcus aureus bacteremia among individuals with and without end-stage renal disease: a Danish, population-based cohort study. BMC Infect Dis 15: p6.
[Crossref], [Googlescholar], [Indexed]
- Oumokhtar B, Elazhari M, Timinouni M, Bendahhou K, Bennani B, et al. (2013) Staphylococcus aureus nasal carriage in a Moroccan dialysis center and isolates characterization. Hemodial Int 17: 542-547.
[Crossref], [Googlescholar], [Indexed]
- Scheuch M, Freiin von Rheinbaben S, Kabisch A, Engeßer J, Ahrendt S, et al. (2019) Staphylococcus aureus colonization in hemodialysis patients: a prospective 25 months observational study. BMC Nephrol 20: p153.
[Crossref], [Googlescholar], [Indexed]
- Nielsen J, Ladefoged SD, Kolmos HJ. (1998) Dialysis catheter-related septicaemia--focus on Staphylococcus aureus septicaemia. Nephrol Dial Transplant 13: 2847-2852.
[Crossref], [Googlescholar], [Indexed]
- San-Juan R, Perez-Montarelo D, Viedma E, Lalueza A, Fortún J, et al. (2017) Pathogen-related factors affecting outcome of catheter-related bacteremia due to methicillin-susceptible Staphylococcus aureus in a Spanish multicenter study. Eur J Clin Microbiol Infect Dis 36: 1757-1765.
[Crossref], [Googlescholar], [Indexed]
- Lakhundi S, Zhang K. (2018) Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin Microbiol Rev 31: p00020-18.
[Crossref], [Googlescholar], [Indexed]
- Harmsen D, Claus H, Witte W, Rothgänger J, Claus H, et al. (2003)Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J Clin Microbiol 41: 5442-5448.
[Crossref], [Googlescholar], [Indexed]
- Erikstrup LT, Dinh KM, Andersen PS, Leo Skov R, Kaspersen KA, et al. (2019) Cohort description: The Danish Blood Donor Staphylococcus aureus Carriage Study. Clin Epidemiol 11: 885-900.
[Crossref], [Googlescholar], [Indexed]
- Neidhart S, Zaatreh S, Klinder A, Redanz S, Spitzmüller R, et al. (2018) Predictors of colonization with Staphylococcus species among patients scheduled for cardiac and orthopedic interventions at tertiary care hospitals in north-eastern Germany-a prevalence screening study. Eur J Clin Microbiol Infect Dis 37: 633-641.
[Crossref], [Googlescholar], [Indexed]
- Wang IK, Chang YC, Liang CC, Chuang FR, Chang CT, et al. (2012) Bacteremia in hemodialysis and peritoneal dialysis patients. Intern Med 51: 1015-1021.
[Crossref], [Googlescholar], [Indexed]
- Lacoma A, Edwards AM, Young BC, Dominguez J, Prat C, et al. (2019) Cigarette smoke exposure redirects Staphylococcus aureus to a virulence profile associated with persistent infection. Sci Rep 9: p10798.
[Crossref], [Googlescholar], [Indexed]
- Chaudry MS, Gislason GH, Kamper AL, Rix M, Larsen AR , et al. (2019) Increased risk of Staphylococcus aureus bacteremia in hemodialysis-A nationwide study. Hemodial Int 23: 230-238.
[Crossref], [Googlescholar], [Indexed]
- Glerup R, Svensson M, Jensen JD, Christensen JH. (2019) Staphylococcus aureus Bacteremia Risk in Hemodialysis Patients Using the Buttonhole Cannulation Technique: A Prospective Multicenter Study. Kidney Medicine 1: 263-270.
[Crossref], [Googlescholar], [Indexed]
- Sharma-Kuinkel BK, Wu Y, Tabor DE, Mok H, Sellman BR, et al. (2015) Characterization of alpha-toxin hla gene variants, alpha-toxin expression levels, and levels of antibody to alpha-toxin in hemodialysis and postsurgical patients with Staphylococcus aureus bacteremia. J Clin Microbiol 53: 227-236.
[Crossref], [Googlescholar], [Indexed]
- McNicholas S, Fe Talento A, O'Gorman J, Hannan MM, Lynche m, t al.(2017) Reduced pro-inflammatory responses to Staphylococcus aureus bloodstream infection and low prevalence of enterotoxin genes in isolates from patients on haemodialysis. Eur J Clin Microbiol Infect Dis 36: 33-42.
[Crossref], [Googlescholar], [Indexed]
- Verhoeven PO, Gagnaire J, Haddar CH, Grattard F, Thibaudin D, et al.(2016) Identifying Hemodialysis Patients With the Highest Risk of Staphylococcus aureus Endogenous Infection Through a Simple Nasal Sampling Algorithm. Medicine (Baltimore) 95: p3231
[Crossref], [Googlescholar], [Indexed]
- Nouwen JL, Fieren MW, Snijders S, Verbrugh HA, van Belkum A. (2005) Persistent (not intermittent) nasal carriage of Staphylococcus aureus is the determinant of CPD-related infections. Kidney Int 67: 1084-1092.
[Crossref], [Googlescholar], [Indexed]
- Fowler VG, Jr., Nelson CL, McIntyre LM, Kreiswirth BN, Monk A, et al. (2007) Potential associations between hematogenous complications and bacterial genotype in Staphylococcus aureus infection. J Infect Dis 196: 738-747.
[Crossref], [Googlescholar], [Indexed]
- Lilje B, Rasmussen RV, Dahl A, Stegger M, Skov RL, et al. (2017) Whole-genome sequencing of bloodstream Staphylococcus aureus isolates does not distinguish bacteraemia from endocarditis. Microbial genomics 3: p000138.
[Crossref], [Googlescholar], [Indexed]
- Austin ED, Sullivan SS, Macesic N, Mehta M, Miko BA, et al. (2019) Reduced mortality of Staphylococcus aureus bacteremia in a retrospective cohort study of 2139 patients: 2007 - 2015. Clin Infect Dis. 70: 1666-1674.
[Crossref], [Googlescholar], [Indexed]
- Lai CF, Liao CH, Pai MF, Chu FY, Hsu SP, et al. (2011) Nasal carriage of methicillin-resistant Staphylococcus aureus is associated with higher all-cause mortality in hemodialysis patients. Clin J Am Soc Nephrol 6: 167-174.
[Crossref], [Googlescholar], [Indexed]
- Grunewald T, Lindner M, Weiss S, Ruf I, Treutler T, et al. (2013) Staphylococcus colonization, mortality and morbidity in hemodialysis patients: 10 years of observation. Int J Hyg Environ Health 216: 751-754.
[Crossref], [Googlescholar], [Indexed]
- Price A, Sarween N, Gupta I, Baharani J. (2015) Meticillin-resistant Staphylococcus aureus and meticillin-susceptible Staphylococcus aureus screening in a cohort of haemodialysis patients: carriage, demographics and outcomes. J Hosp Infect 90: 22-27.
[Crossref], [Googlescholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences