Serum Soluble (Pro) Renin Receptor Level is Increased in Association with Impaired Renal Function in Patients with Autosomal Dominant Polycystic Kidney Disease
Tokiko Miyaoka, Satoshi Morimoto, Hiroshi Kataoka, Toshio Mochizuki, Ken Tsuchiya, Atsuhiro Ichihara and Kosaku Nitta
DOI10.21767/2472-5056.100053
Tokiko Miyaoka1, Satoshi Morimoto2, Hiroshi Kataoka1, Toshio Mochizuki1, Ken Tsuchiya1, Atsuhiro Ichihara2 and Kosaku Nitta1*
1Department of Medicine IV, Tokyo Women’s Medical University, Tokyo, Japan
2Department of Medicine II, Tokyo Women’s Medical University, Tokyo, Japan
- *Corresponding Author:
- Kosaku Nitta
Department of Medicine IV, Tokyo Women’s Medical University, 8-1 Kawada-cho, Shinjuku-ku, Tokyo 162-8666, Japan
Fax: 81353794360
Tel: 81333538111
E-mail: knitta@twmu.ac.jp
Received date: January 19, 2018; Accepted date: February 05, 2018; Published date: February 12, 2018
Citation: Miyaoka T, Morimoto S, Kataoka H, Mochizuki T, Tsuchiya K, et al. (2018) Serum Soluble (Pro) Renin Receptor Level is Increased in Association with Impaired Renal Function in Patients with Autosomal Dominant Polycystic Kidney Disease. J Clin Exp Nephrol Vol 3:2. DOI: 10.21767/2472-5056.100053
Copyright: © 2018 Miyaoka T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The aim of this study was to examine the relationship between the serum soluble prorenin receptor [(P)RR] levels and various clinical parameters of autosomal dominant polycystic kidney disease (ADPKD) patients.
Methods: A total of 79 patients with ADPKD were enrolled and their serum soluble (P)RR levels were measured with ELISA kits. Serum creatinine (Cr), urea nitrogen (UN), uric acid (UA), and albumin levels, blood haemoglobin (Hb) concentration, plasma renin activity (PRA), plasma aldosterone concentration (PAC), and urinary protein/Cr ratio were also measured.
Results: Univariate analyses showed positive correlations between soluble (P)RR levels and age (P=0.02, r=0.27), serum Cr (P<0.0001, r=0.50), UN (P<0.0001, r=0.45), UA (P=0.04, r=0.23) levels and urinary protein/Cr ratio (P<0.001, r=0.42), negative correlations with estimated glomerular infiltration rate (eGFR) (P<0.0001, r=-0.49) and Hb concentrations (P=0.02, r=-0.26), and no correlation with PRA or PAC levels. Soluble (P)RR levels were positively correlated with total kidney volume (TKV, P<0.01, r=0.35). There was no correlation between soluble (P)RR levels and prescription of renin-angiotensin system inhibitors. Finally, multiple regression analyses showed that serum soluble (P)RR concentration was significantly associated with eGFR after correction TKV.
Conclusions: The serum soluble (P)RR levels correlated with their kidney function of the ADPKD patients, suggesting that soluble (P)RR may be involved in kidney injury and promote the progression of renal dysfunction of ADPKD patients.
Keywords
Polycystin kidney disease; Soluble (pro) renin receptor; Renal function; Total kidney volumes
Introduction
Autosomal-dominant polycystic kidney disease (ADPKD) is caused by mutations in PKD1 or PKD2, the genes encoding polycystin-1 and -2. ADPKD affects between 1 in 500 to 1000 people and is characterized by the slow development of large fluid-filled cysts in the kidneys [1]. The cysts cause dramatic enlargement of the kidneys and compromise the functional integrity of the remaining parenchyma. Renal function begins to decline in the fourth decade of life, when the glomerular filtration rate (GFR) starts decrease by 4.4 to 5.9 ml/min per year, and significant impairments of renal function develop by late middle age [2]. The declining GFR rate has been found to be relatively constant in Japanese ADPKD patients and not to be correlated with age or with GFR after adolescence [3].
Patients with ADPKD develop hypertension earlier than those with essential hypertension and early-onset hypertension is the major predictor of renal outcome in ADPKD [4]. Renin, angiotensinogen, and angiotensin (AT)-II are produced in cysts and dilated tubules, and are detected in cyctic fluid [5]. Treatment of hypertension by renin-angiotensin system (RAS) inhibitors is effective in delaying decrease of renal function [6,7].
The serum level of prorenin, a precursor of renin, is elevated up to several hundred fold in diabetes patients [8] and is a predictive marker of the onset of microvascular damage. In particular, young diabetic patients with high serum prorenin levels are at increased risk of developing retinopathy and microalbuminuria [9,10]. The (pro)renin receptor [(P)RR] is a specific receptor for renin and prorenin and was identified as a member of the renin-angiotensin system (RAS) by Nguyen et al. [11,12]. (P)RR is a 350-aminoacid protein with a single trans membrane domain and is widely expressed in various organs, including the brain, heart, and kidney [12]. It is possible that increased (P)RR expression may have a role in the pathogenesis of ADPKD via increased intrarenal RAS.
However, despite recent elucidation of the disconnected ligand binding sites of (P)RR and their presence in serum and urine in the form of soluble (P)RR and a possible role of serum soluble (P)RR concentration as a biomarker to reflect the tissue RAS status, the pathophysiology and clinical significance of serum prorenin and soluble (P)RR in ADPKD patients are unclear despite recent progress in kidney disease research [13]. Although it is anticipated that serum soluble (P)RR concentration might be associated with renal function, deterioration of renal function, and total kidney volume (TKV) in these patients, these issues remain to be determined. Therefore, the aim of this study to examine the pathophysiological roles of serum soluble (P)RR in ADPKD patients by investigating the influence of their age, clinical parameters, including renal function, progression of renal dysfunction, and TKV on their serum soluble (P)RR levels.
Materials and Methods
Study population
Patients with previously diagnosed ADPKD or a positive family history for ADPKD had been screened for enrolment, and the diagnosis of ADPKD was based on ultrasonography diagnostic criteria [14]. The ADPKD patients were classified according to estimated GFR (eGFR) calculated by using the formula devised for the Japanese population-eGFR in mL/min/1.73 m2=194 × serum creatinine-1.094 × age-0.287 × 0.739 (for women), as previously described [15]. The rate of change in eGFR (ΔeGFR) was calculated as [(followed eGFR after 5 years-baseline eGFR)/ baseline eGFR].
The exclusion criteria were: previous dialysis or kidney transplantation, any type of heart failure, liver cirrhosis, past or current malignancy, pregnancy, acute coronary syndrome or ischemic stroke within 3 months prior to enrolment in the study, initiation of dialysis therapy within 6 months after the diagnosis of ADPKD, immunosuppressant use, and life expectancy less than 1 year. Ultimately, 79 patients were enrolled in the study. The study was approved (No. 2409) by the institutional ethics committee and all participants gave written informed consent to participation in the study. The study was carried out in accordance with the ethical principles of the Declaration of Helsinki.
Measurements of biochemical parameters and TKV
The following baseline demographic and clinical data were recorded: age, gender, body mass index (BMI), and blood pressure (BP). Blood samples for laboratory measurements were drawn in the morning after an at least 8 h overnight fast, and after centrifuging immediately at 1,500 g and 4 for 10 min, the supernatants were stored in aliquots at -80. Blood hemoglobin (Hb) concentrations were analyzed separately.
Laboratory measurements included serum creatinine (Cr), albumin, urea nitrogen (UN), uric acid (UA), total cholesterol, and triglyceride, and the measurements were made by using standard methods. Plasma renin activity (PRA) and plasma aldosterone concentration were measured by radioimmunoassay. We also calculated the urinary protein/Cr ratio. Total kidney volume (TKV) was measured by highresolution magnetic resonance imaging based on volumetric measurements of cross-sectional images, as described in the report by the CRISP study [16].
Serum soluble (P)RR levels were measured by using an enzyme-linked immunosorbent assay (ELISA) kit (Takara Bio Inc., Otsu City, Japan) that consisted of a solid-phase sandwich ELISA with antibodies highly specific for each protein [17,18]. The relationships between initial serum soluble (P)RR data, and follow-up clinical parameters on kidney function were then evaluated.
Statistical analysis
All data whose values showed a normal distribution are expressed as the mean ± standard deviation (SD). Single linear univariate correlations were evaluated using Pearson’s correlation coefficient. Groups were compared using one-way analysis of variance, Dunnett’s test, and the chi-squared test, as appropriate.
Multiple regression analyses with serum soluble (P)RR levels and eGFR as dependent variables were conducted by a least squares method and a stepwise forward selection method, respectively. Statistical significance was concluded to be present when the P value was <0.05. All statistical analyses were performed using the JMP (Ver. 9) statistical program.
Results
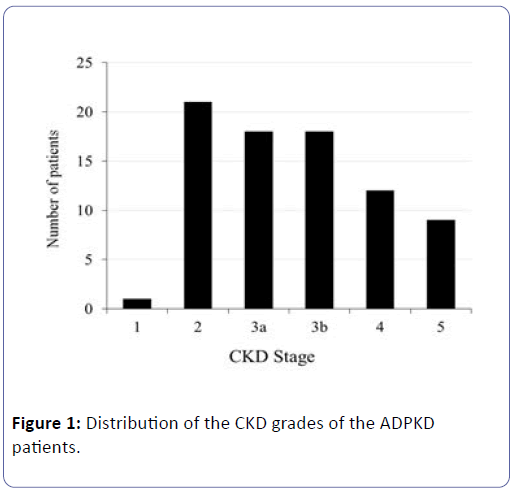
Subjects were subdivided into 5 grades according to their eGFR, and the numbers of patients according to the grade are shown in (Figure 1).
The clinical characteristics of the 79 ADPKD patients are shown in (Table 1). Their mean age was 51 ± 12 years, and the following were their mean serum Cr level 1.7 ± 1.4 mg/dL, UN 24.9 ± 16.8 mg/dL, UA 6.0 ± 1.2 mg/dL, and eGFR 45.7 ± 23.6 mL/min/1.73 m2, and their urinary protein/Cr ratio was 0.1 ± 0.2 mg/g Cr.
| Patient characteristics | |
|---|---|
| Male, n (%) | 33 (42.0) |
| Age (years) | 51 ± 12 |
| Body mass index (kg/m2) | 21.4 ± 2.3 |
| Systolic blood pressure (mmHg) | 123 ± 12 |
| Diastolic blood pressure (mmHg) | 76 ± 8 |
| Mean blood pressure (mmHg) | 91 ± 8 |
| Laboratory data | |
| Hemoglobin (g/dl) | 12.6 ± 1.7 |
| Albumin (g/dl) | 4.3 ± 0.4 |
| Urea nitrogen (mg/dl) | 24.9 ± 16.8 |
| Triglyceride (mg/dl) | 129 ± 78 |
| Total cholesterol (mg/dl) | 160 ± 54 |
| LDL cholesterol (mg/dl) | 107 ± 23 |
| HDL cholesterol (mg/dl) | 66 ± 18 |
| Creatinine (Cr, mg/dl) | 1.7 ± 1.4 |
| eGFR (ml/min/1.73m2) | 45.7 ± 23.6 |
| ΔeGFR 5 years rate of change*1 (%) | -25.2 ± 22.5 |
| Uric acid (mg/dl) | 6.0 ± 1.2 |
| HbA1c (%) | 5.6 ± 0.3 |
| Serum soluble (P)RR (ng/ml) | 20.3 ± 4.4 |
| Plasma renin activation (ng/ml/h) | 4.38 ± 5.91 |
| Plasma aldosterone concentration (pg/ml) | 213.8 ± 161.1 |
| Urinary protein to creatinine ratio (g/g ÃÆââ¬Â¹Ãââ⢠Cr) | 0.1 ± 0.2 |
| Urinary occult blood index*2 | 1.1 ± 0.3 |
| Total kidney volume (ml) | 1292 ± 679 |
| Anti-hypertensive agents | |
| ACE inhibitor, n (%) | 5 (6.3) |
| ARB, n (%) | 50 (63.3) |
| Renin inhibitor, n (%) | 10 (12.7) |
| CCB, n (%) | 18 (22.8) |
*1: (At registration–5 years ago)/5 years ago *2: Count of red blood cells in the urine (0-4/HF=0, 5-9/HF=1, 10-29/HF=2, 30-49/HF=3, 50- 99/HF=4, >100/HF=5)
Table 1: Clinical characteristics of study population.
The serum soluble (P)RR levels were analyzed for Cr, eGFR, ΔeGFR , UN, UA, age, and the urinary protein/Cr ratio (Table 2).
| Variables | R | P-value |
|---|---|---|
| Male | 0.41 | |
| Age | 0.27 | 0.02 |
| Body mass index | 0.01 | 0.91 |
| Systolic blood pressure | 0.09 | 0.45 |
| Diastolic blood pressure | 0.03 | 0.82 |
| Mean blood pressure | 0.06 | 0.60 |
| Hemoglobin | -0.26 | 0.02 |
| Albumin | -0.12 | 0.29 |
| Triglyceride | 0.17 | 0.18 |
| Total cholesterol | 0.29 | 0.41 |
| Ldl cholesterol | 0.19 | 0.13 |
| Hdl cholesterol | -0.28 | 0.04 |
| Urea nitrogen | 0.45 | <0.0001 |
| Creatinine (Cr) | 0.5 | <0.0001 |
| eGFR | -0.49 | <0.0001 |
| 5 years rate of change | -0.44 | <0.01 |
| Uric acid | 0.23 | 0.04 |
| Hb A1c | -0.06 | 0.72 |
| Plasma renin activity | -0.10 | 0.48 |
| Plasma aldosterone concentration | -0.06 | 0.69 |
| Urinary protein to creatinine ratio | 0.42 | <0.001 |
| Urinary occult blood index | 0.02 | 0.86 |
| Total kidney volume | 0.35 | <0.01 |
Table 2: Correlation between serum soluble (P)RR and clinical parameters.
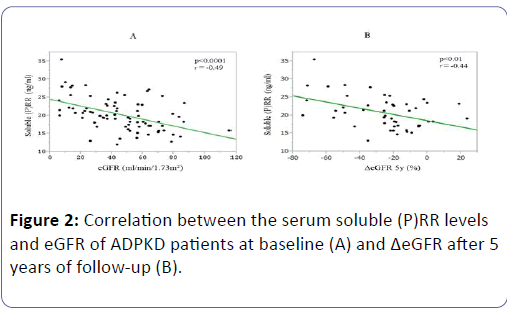
Their soluble (P)RR levels were positively correlated with their serum levels of Cr (P<0.0001, r=0.50), UN (P<0.0001, r=0.45), and UA (P=0.04, r=0.23), and with their age (P=0.02, r=0.27), and negatively correlated with their Hb concentrations (P=0.02, r=-0.26), eGFR (P<0.0001, r=-0.49) (Figure 2A), and ΔeGFR (P<0.01, r=-0.44) (Figure 2B).
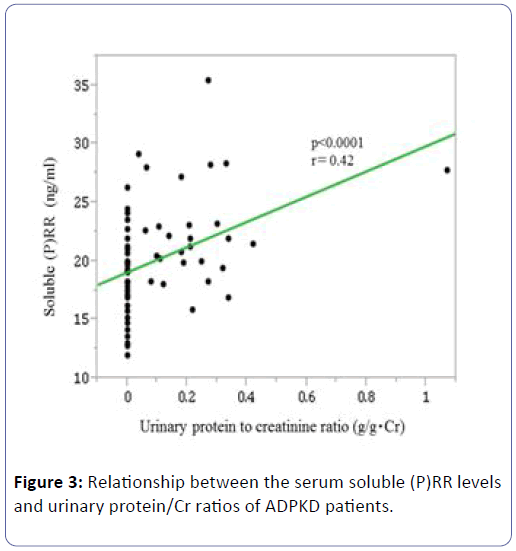
Their soluble (P)RR levels were positively correlated with their urinary protein/Cr ratios (P<0.0001, r=0.42) (Figure 3), but they were not significantly correlated with PRA (P=0.48, r=-0.10) or PAC (P=0.69, r=-0.06).
Investigation of the relationships between soluble (P)RR levels and use of antihypertensive drugs showed no significant differences between soluble (P)RR levels according to whether patients were treated with antihypertensive drugs, including angiotensin-converting enzyme inhibitors (ACEIs, P=0.65), angiotensin receptor blockers (ARBs, P=0.77), and calcium channel blockers (P=0.10) (Table 3).
| Variables | P-value |
|---|---|
| RAS inhibitor | 0.65 |
| ACE inhibitor | 0.65 |
| ARB | 0.77 |
| Renin inhibitor | 0.43 |
| CCB | 0.10 |
RAS: Renin-angiotensin system; ACE: Angiotensin-converting enzyme; ARB: Angiotensin receptor blocker; CCB: Calcium channel blocker
Table 3: Relationship between soluble (P)RR and use of antihypertensive agents.
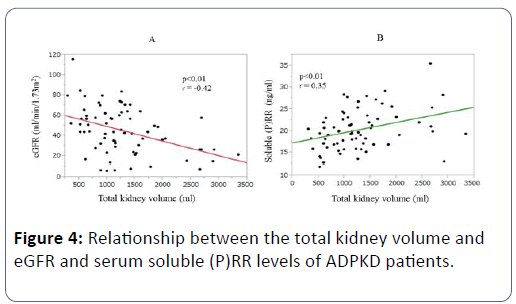
The TKVs were negatively correlated with eGFR (P<0.01, r=-0.42) (Figure 4A). Interestingly, the soluble (P)RR levels of the ADPKD patients were positively correlated with their TKVs (P<0.01, r=0.35) (Figure 4B), and the multiple regression analysis showed that their TKVs were negatively correlated with eGFR (P<0.01, r=-0.34) but not with their serum soluble (P)RR levels (P=0.15, r=0.18) (Table 4).
| Variables | β | P-value |
|---|---|---|
| Soluble (P)RR | 0.18 | 0.15 |
| eGFR | -0.34 | <0.01 |
Table 4: Multiple regression analysis for correlation with total kidney volume.
Discussion
The results of this study showed that the soluble (P)RR levels of the ADPKD patients were positively correlated with their serum Cr, UN, and UA levels, and with their urinary protein/Cr ratios, and negatively correlated with their eGFR and Hb values. Notably, the simple regression analyses showed that their soluble (P)RR levels were significantly correlated with their TKVs. Interestingly, the basal soluble (P)RR levels were negatively correlated with their ΔeGFR at 5 years. In addition, serum soluble (P)RR concentration was significantly negatively associated with eGFR independent of other factors including TKV. To the best of our knowledge, this is the first study to demonstrate relationships between the serum soluble (P)RR levels of ADPKD patients and their disease severity and progression, TKVs , and antihypertensive therapy.
The expression of (P)RR in the kidneys suggested that prorenin may be able to generate angiotensin (AT)-I from angiotensinogen by binding to (P)RR. If it does, the subsequent overproduction of AT-II may then stimulate inflammation and fibrosis in the renal interstitial space. Alternatively, (P)RR may play a role in the tubular transport of water and electrolytes, including H[19]. A previous study reported observing increased (P)RR expression in the remnant kidneys of 5/6 nephrectomised rats [20]. A study conducted by Ichihara et al. showed that a (P)RR blocker administered to rats with streptozotocin-induced diabetes or spontaneous hypertension was able to inhibit the development and progression of end-organ damage and that it had a greater benefit than conventional inhibitors in relation to the RAS in diabetic AT-II type 1a receptor-deficient mice [21].
(P)RR has recently been shown to function as an adaptor between Wnt receptors in a renin-independent manner and the vacuolar H-adenosine triphosphatase (V–ATPase) complex, and (P)RR and V–ATPase are required to mediate Wnt signalling [22]. If soluble (P)RR levels reflect (P)RR levels in the kidneys, the increased levels of soluble (P)RR might reflect activation of renal (P)RR to promote the progression of kidney injury and fibrosis in ADPKD. However, the increased soluble (P)RR levels may have simply been the results of decreased GFR. A recent report suggested that soluble (P)RR in the renal medulla is stimulated during AT-II-dependent hypertension and that this form of the (P)RR is secreted into the tubular fluid [23]. This finding indicates that soluble (P)RR levels might be increased as a result of decreased GFR. Although (P)RR-mediated signalling is thought to be involved in the development of glomerular diseases, (P)RR expression in podocytes and its function in autophagy suggests a physiological rather than a pathological role in the kidneys [24]. The function of (P)RR in ADPKD remains unknown, but based on our findings in this study, we hypothesized that elevated serum (P)RR levels may imply increase expression of (P)RR in the kidneys to promote the deterioration of renal function in ADPKD.
While relatively few studies have described the regulatory mechanisms of (P)RR expression, the results of a recent study suggest that (P)RR expression may be regulated by renin through a negative feedback loop [25]. In fact, up-regulation of renal renin and down regulation of renal (P)RR have been observed after ACE inhibition and a change to a low-salt diet [26]. Renin down-regulates (P)RR expression by a process involving the transcription factor promyelocytic zinc finger protein [24]. Whether the increased serum (P)RR levels in ADPKD are the result of a negative feedback signal in the form of decreased intrarenal renin remains to be clarified [26].
Hamada et al. have recently measured the serum soluble (P)RR levels of 374 patients with chronic kidney disease (CKD) [27], and shown that they were positively correlated with their serum Cr, UN, and UA levels, urinary protein/Cr ratio, and negatively correlated with their eGFRs and Hb concentrations. These results are consistent with findings in the present study. The results obtained by Hamada et al. with respect to antihypertensive drugs showed that the soluble (P)RR levels of the group with an ARB were significantly lower than in the group not treated with an ARB [27]. Their results for the relationship between basal soluble (P)RR levels and renal dysfunction showed that soluble (P)RR levels were inversely associated with ΔeGFR, suggesting that soluble (P)RR may be involved in kidney injury and promote the progression of CKD.
The plasma soluble (P)RR levels of healthy subjects have been found not to correlate with their renin, prorenin, or aldosterone levels [28], but the serum soluble (P)RR levels have been shown to be negatively correlated with eGFR independent of age, blood pressure, and glucose metabolism in essential hypertension and normotensive subjects [29]. In addition, the serum soluble (P)RR levels have been shown to be positively correlated with their urinary levels of angiotensinogen, a biomarker of intrarenal RAS [29,30]. In human kidneys with end-stage renal disease (ESRD), intrarenal (P)RR was immunostained mainly in the tubules, suggesting a possible contribution of increased renal (P)RR expression to the elevated serum soluble (P)RR levels in ESRD [31]. The above findings suggest that soluble (P)RR might reflect intrarenal RAS status. It is proposed that over activity of the intrarenal RAS might be associated with the sustained increases in intratubular AT-II concentrations, whereby the intrarenal RAS activation induces cyst enlargement in ADPKD [5].
In the present study, multiple regression analysis revealed that serum soluble (P)RR concentration was significantly associated with eGFR after correction for other factors including TKV. This finding is noteworthy because it may suggest that renal dysfunction is due not only to cyst formation but also to increase AT-II synthesized by (P)RR in non-cystic renal parenchyma of the kidney. It is expected that intervention to inhibit intrarenal (P)RR expression may be beneficial in protecting renal function in ADPKD, although this presumption needs to be tested by further examinations.
The present study had several limitations. First, we did not include healthy volunteers and were therefore unable to measure the soluble (P)RR levels of healthy subjects for comparison. Age may also be associated with changes in soluble (P)RR levels, and we hope to investigate the possible association in future studies. Second, our study was retrospective and crosssectional in nature, and we did not investigate for possible changes in soluble (P)RR levels over time. Monitoring the soluble (P)RR levels of ADPKD patients over time would be useful as a means of assessing its potential role as a biomarker of kidney disease progression. Based on the results of this study, we would like to determine the relationship between soluble (P)RR and the (P)RR levels in kidney tissue in a future prospective study. Third, we were unable to determine prorenin activation levels, because the samples had been preserved before testing. Such information would have provided further insight into the role of soluble (P)RR in the RAS.
In summary, the results of this study showed that the serum soluble (P)RR levels are correlated with their renal function of ADPKD patients. We speculate that elevated serum soluble (P)RR level might reflect increased expression of renal (P)RR which might promote the progression of kidney injury in ADPKD patients. The results of previous studies in animal disease models in which (P)RR function was blocked have suggested that (P)RR is involved in the pathogenesis of CKD, and that prorenin/ (P)RR is involved in the onset and progression of organ injury. Thus, further study of prorenin/(P)RR in ADPKD may clarify disease progression and the potential role of soluble (P)RR as a marker of renal disease progression.
Acknowledgments
We thank the medical staff of Department of Medicine, Kidney Center, Tokyo Women’s Medical University Hospital for collecting the laboratory samples from the ADPKD patients.
Statements of Ethics
All participants provided written informed consent. The study was supported by grants from Otsuka Pharmaceutical Co., Chugai Pharmaceutical Co., Kyowa Hakko Kirin Co., MSD Co. and JMS Co.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- Ong AC, Devuyst O, Knebelman B, Walz G (2015) ERA-EDTA Working Group for Inherited Kidney Disease: Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 385: 1993-2002.
- Torres VE, Harris Y (2009) Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int 76: 149-168.
- Higashihara E, Horie S, Muto S, Mochizuki T, Nishio S, et al. (2012) Renal disease progression in autosomal polycystic kidney disease. Clin Exp Nephrol 16: 622-628.
- Chapman AB, Gabow PA (1997) Hypertension in autosomal dominant polycystic kidney disease. Kidney Int Suppl 61: S71-S73.
- Loghman-Adham M, Soto CE, Inagami T, Cassis L (2004) The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol 287: F775-F788.
- Nutahara K, Higashihara E, Horie S, Kamura K, Tsuchiya K, et al. (2005) Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron Clin Pract 99: c18-c23.
- Sans-Atxer L, Torra R, Fernandez-Llama P (2013) Hypertension in autosomal-dominant polycystic kidney disease (ADPKD). Clin Kidney J 6: 457-463.
- Derkx FH, Schalekamp MA (1988) Human prorenin: pathophysiology and clinical implications. Clin Exp Hypertens A 10: 1213-1225.
- Luetscher JA, Kraemer FB, Wilson DM, Schwartz HC, Bryer-Ash M (1985) Increased plasma inactive renin in diabetes mellitus. A marker of micro vascular complications. N Engl J Med 312: 1412-1417.
- Wilson DM, Luetscher JA (1990) Plasma prorenin activity and complications in children with insulin-dependent diabetes mellitus. N Engl J Med 323: 1101-1106.
- Nguyen G, Delarue F, Berrou J, Rondeau E, Sraer JD (1996) Specific receptor bindingofreninonhuman mesangial cells in culture increases plasminogen activator-1 antigen. Kidney Int 50: 1897-1903.
- Nguyen G, Delarue F, Burckle´ C, Bouzhir L, Giller T, et al. (2002) Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417-1427.
- Oshima Y, Morimoto S, Ichihara A (2014) Roles of the (pro)renin receptor in the kidney. World J Nephrol 3: 302-307.
- Mochizuki T, Tsuchiya K, Nitta K (2013) Autosomal polycystic kidney disaease: recent advances in pathogenesis and potential therapies. Clin Exp Nephrol 17: 317-326.
- Imai E, Matsuo S, Makino H. Watanabe T, Akizawa T, et al. (2010) Chronic Kidney Disease Japan Cohort study: baseline characteristics and factors associated with causative diseases and renal function. Clin Exp Nephrol 14: 558-570.
- Bae KT, Commean PK, Lee J (2000) Volumetric measurement of renal cysts and parenchyma using MRI: phantoms and patients with polycytic kidney disease. J Compu Assist Tomogr 24: 614-619.
- Watanabe N, Bokuda K, Fujiwara T, Suzuki T, Mito A, et al. (2012) Soluble (pro)renin receptor and blood pressure during pregnancy: a prospective cohort study. Hypertens 60: 1250-1256.
- Amari Y, Morimoto S, Nakajima F, Ando T, Ichihara A (2016) Serum soluble (pro)renin receptor levels in maintenance hemodialysis patients. PLoS One 11: e0158068.
- Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, et al. (2009) The (Pro)renin receptor: site specific and functional linkage to the vacuolar HÃÆïÃâüÃâââ¬Â¹–ATPase in the kidney. Hypertens 54: 261-269.
- Hirose T, Mori N, Totsune K, Morimoto R, Maejima T, et al. (2010) Increased expression of (pro)renin receptor in the remnant kidneys of 5/6 nephrectomized rats. Regul Pept 159: 93-99.
- Ichihara A, Itoh H, Inagami T (2008) Critical roles of (pro)renin receptor-bound prorenin in diabetes and hypertension: sallies into therapeutic approach. J Am Soc Hypertens 2: 15-19.
- Cruciat CM, Ohkawara B, Acebron SP, Karaulanov E, Reinhard C, et al. (2010) Requirement of prorenin receptor and vacuolar H-ATPase-mediated acidification for Wnt signaling. Science; 327: 459-463.
- Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC (2011) Soluble form of the (pro)renin receptor is augmented in the collecting duct and urine of chronic angiotensin II-dependent hypertensive rats. Hypertens 57: 859-864.
- Ichihara A (2012) (Pro)renin receptor and autophagy in podocytes. Autophagy 8: 271-272.
- Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, et al. (2006) A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res 99: 1355-1366.
- Nguyen G, Contrepas A (2008) Physiology and pharmacology of the (pro)renin receptor. Curr Opin Pharmacol 8: 127-1232.
- Hamada K, Taniguchi Y, Shimamura Y, Inoue K, Ogata K, et al. (2013) Serum level of soluble (pro)renin receptor is modulated in chronic kidney disease. Clin Exp Nephrol 17: 848-856.
- Nguyen G, Blanchard A, Curis E, Bergerot D, Chambon Y, et al (2014) Plasma soluble (pro)renin recptor is independent of plasma renin, prorenin, and aldosterone concentrations but is affected by ethnicity. Hypertens 63: 297-302.
- Morimoto S, Ando T, Niiyama M, Seki Y, Yoshida N, et al. (2014) Serum soluble (pro)renin receptor levels in patients with essential hypertension. Hypertens Res 37: 642-648.
- Kobori H, Alper AB Jr, Shenava R, Katsurada A, Saito T, et al. (2009) Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system in hypertensive patients. Hypertens 53: 344-350.
- Takahashi K, Yamamoto H, Hirose T, Hiraishi K, Shoji I, et al. (2010) Expression of (pro)renin receptor in human kidneys with end-stage kidney disease due to diabetic nephropathy. Peptides 31:1405-1408.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences