Research Advances on the Genetic Mechanism of Diabetic Nephropathy
Miao Ti, Lingyu Xue, Qiao Yin, Shan Shao, Jiao Cai and Wen Zhang
DOI10.21767/2472-5056.100029
Miao Ti, Lingyu Xue*, Qiao Yin, Shan Shao, Jiao Cai and Wen Zhang
Department of Nephrology, The Affiliated Hospital of Taishan Medical Unviersity, PR China
- *Corresponding Author:
- Lingyu Xue
Department of Nephrology, The Affiliated Hospital of Taishan Medical Unviersity
No.706 Taishan Street, Taishan Distract, TaiAn City, PR China
Tel: 13854804507
E-mail: xuelingyu000@163.com
Received date: February 21, 2017; Accepted date: March 14, 2017; Published date: March 17, 2017
Citation: Ti M, Xue L, Yin Q, Shao S, Cai J, et al. (2017) Research Advances on the Genetic Mechanism of Diabetic Nephropathy. J Clin Exp Nephrol 2:29. doi: 10.21767/2472-5056.100029
Copyright: © 2017 Ti M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: This article focuses on the genetic mechanisms, with an aim to provide certain theoretical and experimental foundation for the future diagnosis and treatment of diabetic nephropathy. Five common genes were selected in this article including ACE, Klotho, MicroRNA, CERS and VDR.
Methods: To learn about the newest study trends on this topic through searching numerous international and domestic databases (such as CNKI, CBMdisc, PUBMED, EBMase) aiming to obtain meaningful conclusions in order to write a valuable summarization.
Results: These five genes both play important roles in the pathogenesis of diabetic nephropathy: ①The I allele of ACE gene is the protective gene that prevents type 2 diabetes mellitus patients from developing diabetic Nephropathy; ② In diabetic nephropathy, the level of Klotho mRNA and Klotho protein is decreased by the RAAS, TGFβ, PPAγ, and Wnt signaling pathways; ③ miR-29c, which is involved in the regulation of the Spry1/Rho signaling pathway, can be used as a new target for the treatment of diabetic nephropathy; The SNP (rs267734) in the CERS2 gene is associated with an increase in albuminuria among patients with diabetes; The occurrence of diabetic nephropathy is associated with vitamin D receptor gene genetic polymorphisms.
Conclusion: With an ever-increasing access to this subject, we can further our understanding of the pathogenesis of DN and broaden the research field until accomplishing the target of prevention of DN through the creation of new target drug and accurate prediction of disease. Further study about the genetic mechanism of DN especially T1DN remains a vital task.
Keywords
Diabetic nephropathy; Genetic mechanism; Diabetic complications; kidney hypertrophy
Introduction
Among diabetic complications, diabetic nephropathy (DN) has already become the leading cause of end-stage kidney disease (ESKD) [1,2]. DN patients usually present with a “microalbuminuria” symptom after an incubation period, as well as kidney hypertrophy and an increase in GFR. According to statistics, macroalbuminuria occurs in approximately 20-40% of diagnosed diabetic patients within 15-20 years, and around half of these patients will present renal insufficiency within the next 5 years [3]. DN can be divided into five clinical stages according to the course and pathological physiology evolution process of diabetes. Although no clear etiology or pathogenesis for DN has been identified as of recently, it is clearly a complex, multifactorial process. Not only is the onset of DN related to inappropriate changes in renal hemodynamics, activation of protein kinase C, activation of the hexosamine biosynthesis pathway, activation of the aldose reductase pathway and the formation of advanced glycation end products (AGE), but it is also related to abnormal changes of genes structure and function [4,5]. Exploring the genetic mechanisms of diabetic nephropathy has become a hot topic worldwide. Further study about this subject has great clinical significance for the early diagnosis and prevention of DN as well as in delaying progression to the advanced stages. The ultimate goal is to improve the survival rate of diabetic patients. The expression of the genes that are involved in the progression of DN varies, and this essay focuses on five vital genes involved in DN.
ACE
ACE polymorphism
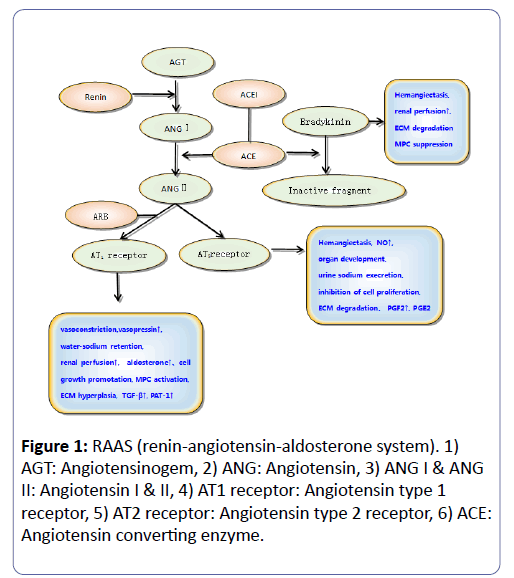
Abnormal renal hemodynamics is one of the important characteristics of DN, and RAAS (renin-angiotensin-aldosterone system) is significantly involved in its regulation. Granted, RAAS genes are thought to be important candidate genes of DN. This system consists of renin, angiotensin II, angiotensin I, angiotensinogen, angiotensin converting enzyme and angiotensin receptors. Interestingly, this system can not only reduce the degradation of the sustained-release atrial natriuretic peptide and nitric oxide generation, but it can also promote oxidative stress, inducing the formation of free radicals and increasing vascular endothelial injury. Conversely, it can also increase vascular blood volume, increase vascular resistance by contracting vessels and further aggravate vascular injury [6,7].
ACE is the key enzyme in the RAAS that catalysis the conversion of angiotensin I to angiotensin II with strong contractile activity [8]. The human ACE gene is located on chromosome 17q23 and is approximately 21 kb in length, including 26 exons and 25 introns. The deletion of the Alu repeat sequence in the sixteenth intron of the 287th BP can lead to the I/D ACE polymorphism. Several studies have explored the correlation between the ACE gene polymorphism and diabetic nephropathy (Figure 1).
ACE (I/D) Gene Polymorphism in DN
Recently, a meta-analysis has been published in Medical Journal by Qin to define the relationship between the ACE gene polymorphism and diabetic nephropathy. A 287-bp insertion/ deletion polymorphism in intron 16 of the ACE genecan be examined by polymerase chain reaction in patients with DN. Usingretrieval systems (PubMed, Google Scholar and FMRS), 39 articles were includedin the study with 18,996 patients before March 20, 2016. Of those patients, 8, 131 had T2DN as a diagnosis; all others were considered T2DM without nephropathy and were regarded as controls. Statistical analysis was performed using Review Manager 5.0 and Stata 12.0 software, and the results demonstratethat the ACE gene polymorphism compared with the D allele can significantly reduce the risk of DN secondary to T2DM, with the OR value of its overall consolidation effect being 0.72 (95% CI 0.64~0.80). In summary, the ACE gene I/D polymorphism is associated with T2DN, but different degrees of genetic susceptibility are apparent due to varying races and regions. Researchers speculate that the I allele is the protective gene that prevents type 2 diabetes mellitus patients from developing diabetic nephropathy. Optimization of the design of the case-control study is needed to verify the relationship between ACE gene polymorphisms and the diabetic nephropathy.
Klotho
Klotho protein and its function
The Klotho (KL) gene, discovered in 1997 by Kuro-o during a spontaneous hypertension study, is associated with senescence and is mainly expressed in the kidney and brain. This experimental animal study shows that the deletion of the Klotho gene can cause a series of human aging symptoms as follows: shortened lifespan, derangement of phosphorus regulation, osteoporosis, and vascular calcification [9].
The expression products of Klotho include two protein isoforms: a membranebound and a secreting type. The high expression of Klotho in the kidney may play a special role in the development of renal disease. A tetramer is constructed by membrane-bound Klotho protein and fibroblast growth factor receptor (FGFRs) that can work as a co-receptor of FGF23 [10]. FGF23 can directly regulate serum calcium and phosphorous levels, as well as indirectly work together with parathyroid hormone during vitamin D metabolism. Soluble a-klotho, which is derived from the proteolytic cleavage of the extracellular portion of the membrane-bound a-klotho, can be measured in blood, urine, and cerebrospinal fluid. Alternatively, soluble aklotho can be generated directly by the alternative splicing of the a-klotho transcript [11]. Soluble Klotho protein functions as paracrine or autocrine hormones, modulating positive interactions by FGF23, including anti-aging, insulin-like growth factors, and the suppression of PTH. Additionally, it works by adjusting ion channels and several pathways that are discussed in the remainder of the study (Figure 2).
The role of Klotho protein in DN
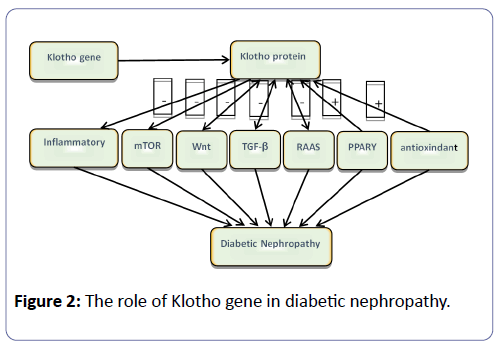
There are various pathways involved in the development and progression of DN, such as the RAAS, TGFβ, PPARγ and Wnt signaling pathways [12]. In diabetic nephropathy, the level of Klotho mRNA and Klotho protein is clearly decreased, and in all probability, connects the interaction between these pathways [13,14].
RAAS: The function of RAAS has been elaborated above. There is no doubt that this system has a pivotal role in the development of DN. Previous research has shown that renal creatinine clearance increased and urinary protein excretion decreased after the Klotho gene was injected into the body of rats with continuous ATII infusion [15]. In conclusion, in diabetic nephropathy, the activation of RAAS inhibits the expression of the Klotho protein in the kidney and the Klotho gene can induce renal damage by inhibiting RAAS.
TGF-β: TGF-β, as a type of fibrosis-inducing factor, not only promotes vascular fibrosis but also promotes the proliferation of vascular tissue in DN patients [16]. Studies performed by Lo CS have shown that high levels of TGF-β1 can aggravaterenal function deterioration and proteinuria, promoting the occurrence and development of diabetic nephropathy [17]. The Klotho protein can prevent TGF-β 1 binding to a TGF-β 2 type receptor, inhibiting the expression of fibrosis markers and TGF-β 1 target genes. Another mechanism is that Klotho can inhibit the TGF-β 1-meditated phosphorylation of Smad2 [18]. Therefore, Klotho protein and TGF-β demonstrate antagonisticeffects by different mechanisms in diabetic nephropathy.
Wnt: In 1982, Nusse and Varmus discovered the Wnt gene in the body of a mouse with breast cancer induced by mouse mammary tumor virus (MMTV) [19]. Under a state of diabetic nephropathy, the activated Wnt/βnt/r as signal pathway is related to mesangial cell apoptosis, podocyte dysfunction and tubular epithelial cell transition leading to renal and interstitial fibrosis [20]. Experimental results have shown that in patients with DN, the loss of the Klotho protein is associated with βwith cia activation, suggesting that there is a negative correlation between the Klotho protein and Wnt pathway [21]. Meanwhile, the Klotho protein can bind to multiple Wnt ligands and inhibit their ability to activate the Wnt signalling pathway [22].
PPAR: Peroxisome proliferator activated receptor (PPAR), a member of the nuclear receptor superfamily, plays an important role in fat metabolism, enhances insulin sensitivity, and impacts tumor and inflammation development [23]. In patients with type 2 diabetes, activation of PPAR can control the effect of insulin resistance, glucose, blood pressure and anti-inflammatory properties, demonstrating a benefit of delaying the progression of diabetic nephropathy [24].
The Klotho gene is the target gene of PPAR. The latest research has revealed that compared to the control group, the expression of Klotho protein in the kidney was significantly decreased in diabetic rats, but when under the treatment of TZD, a PPAR agonist, their Klotho protein level was clearly restored. This study showed that TZD can increase the recovery of Klotho protein and improve DN by activating PPAR.
There were statistically significant correlations between categories of progressively worsening albuminuria and lower plasma a-klotho levels [25]. Currently, preventing deceases in aklotho can be a potential therapeutic strategy for early diabetic nephropathy. For example, angiotensin-converting enzyme inhibitors or ARBs can increase renal Klotho expression, which can decrease the progression of albuminuria in DN [26]. However, long-term prospective studies are needed to elucidate the pathophysiological mechanisms of a-klotho in the development and progression in DN.
MicroRNA
Overview of MicroRNA
MicroRNA is a type of small endogenous, single-stranded RNA molecule, approximately 19-25 nucleotides in length that can react specifically to the target gene (3’ untranslated region) by complementary base-pairing. Mature miRNA can inhibit the translation of target mRNA by pairing with the target mRNA3’- UTR and negatively regulating the expression of genes. Recent studies suggest that miRNA is involved in the pathophysiological process of many diseases such as inflammation, blood glucose and lipid metabolic abnormalities [27]. Additionally, the specific expression of miRNA in renal tissue may be involved in the pathophysiological mechanism of DN. Therefore, further investigation of miRNA will aid in understanding the molecular mechanism of DN and provide new markers for early diagnosis of DN [28].
DN and MicroRNA-29
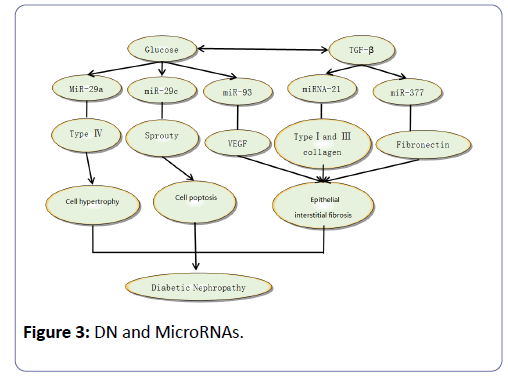
An increasing number of studies demonstrate that miRNAs, including miRNA-29, miRNA-377, miRNA-126, miRNA-145, miRNA-146, miRNA-93, miRNA-21and miRNA-200, are closely related to the pathogenesis of DN (Figure 3). In recent years, miR29 has been proven to be of crucial importance by numerous studies. Several researchers have proved that miR29 is closely related to fibrotic disease, including kidney fibrosis, based on its potential to repress many types of collagen [29]. MiR-29 family is composed of miR-29a, miR-29b and miR-29c, and among these three, miR-29a and miR-29c are involved in the pathogenesis of diabetic nephropathy. Using experimental databases, patients with albuminuria showed a significantly higher comorbidity of diabetic retinopathy, and higher levels of urinary miR-29a were observed compared with those with norm albuminuria [30]. MiR-29a may be regarded as a negative regulator of the collage gene, and high glucose and TGF-β can down-regulate the expression level of miR-29a and promote the expression of Col4a1/Co-l4a2, resulting in matrix collagen aggregation and ultimately leading to albuminuria and diabetic nephropathy. Recent studies have demonstrated that high glucose can induce higher levels of miR29c expressed in podocyte and vascular endothelial cells, which can cause apoptosis and extracellular matrix deposition. Additionally, the over-expression of miR29ccan decrease Spry1 expression and promote Rho kinase activation, while knockout of miR29c can significantly decrease the level of albuminuria and the mesangial matrix-reassembling in db/db mice [31].
In conclusion, miR-29c, which is involved in the regulation of the Spry1/Rho signalling pathway, can be used as a new target for the treatment of diabetic nephropathy.
CERS2ÃÆïÃâüÃâÃâ Ceramide Synthase 2ÃÆïÃâüÃâââ¬Â°
Function of CERS2
Cross sectional studies have identified that genetic variants are associated with eGFR. Therefore, we investigated a theory on whether these variants were also associated with the rate of increase in albuminuria among patients with diabetes [32]. Ceramidase synthase 2 is a hydrolyzing ceramide that can break the bond between fatty acid and sphingosine and then acylates dihydro-sphingosine to form dihydro-ceramide or sphingosine to form ceramide [33]. It is the most abundantly expressed ceramide synthase in animal tissues such as the brain, kidney and spleen [33]. Ceramides are intra-cellular and extra-cellular signalling molecules that play a role in several pathological and physiological processes, including inflammation, diabetes, and angiogenesis [33].
SNP (rs267734) and albuminuria
In 2014, there was a pre-specified investigation about the relationship between the CERS2 gene and albuminuria in diabetic patients by Dov Shiffman in 2014. The substudy consisted of 3,128 patients from ONTARGET and 595 patients from TRANSCEND. Researchers investigated 16 SNPs that were previously reported to be associated with eGFR at the genomewide level. After the estimates including age, ethnicity, sex and principal component of genetic heterogeneity were adjusted for, it was determined that one SNP (rs267734) in the CERS2 gene was associated with an annual rate of increase in albuminuria in diabetic patients, and each risk allele of rs267734 was related to a 50% increased risk of incident albuminuria [32].
Briefly, it was previously found that this SNP in the CERS2 gene is associated with an increase in albuminuria among patients with diabetes [33]. Additional studies are still required regarding whether inhibition of ceramide synthase 2 activity, such as the substitution of glutamic acid to alanine, would inhibit the worsening of albuminuria. Additionally, this may provide a potential research direction to slow the progression from diabetes to diabetic nephropathy by preventing the generation of proteinuria.
VDR (Vitamin D receptorÃÆïÃâüÃâââ¬Â°
VDR and VDR gene
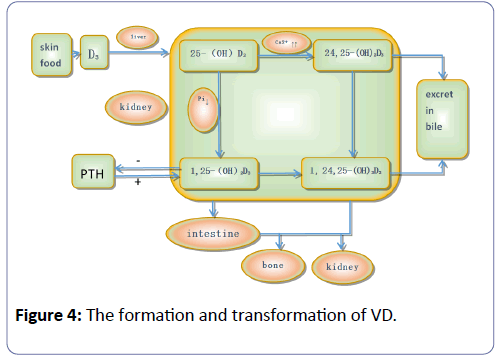
According to epidemiological investigation in diabetic nephropathy patients, the incidence of vitamin D deficiency is as high as 77.5% [34]. Vitamin D is a group of fat-soluble steroid derivatives and works as a secosteroid hormone, which not only has a well-known function of maintaining normal homeostasis of calcium and phosphorus but also has potent non-classical properties such as anti-inflammatory, antioxidant, antiangiogenic, and antiproliferative properties [35,36]. The transduction pathway studies have shown that vitamin D and its analogs can clearly reduce the activity of the renin gene promoter and directly inhibit the expression of renin, which in turn decreases the production of angiotensin II. The molecule 1, 25 (OH) 2D3 negatively regulates the renin-angiotensin system and inhibits the RAS system of renal injury, which plays an important role in the process of diabetic nephropathy (Figure 4).
Vitamin D undergoes biological activity by hydroxyl activity twice in the human body: once in the liver, under the action of hydroxylase as it is transformed into 25 (OH) D3, and then when it is transported to the kidney from the bloodstream to the renal proximal convoluted tubule cells, where the hydroxylation of alpha 1 hydroxylase, 25 (OH) D3 transforms it into 1, 25 (OH) 2D3. It then acquires strong biological activity and can suppress Sertoli cell apoptosis induced by a high sugar environment by blocking the P38 lightning and ERK signal mediated ways.
A large number of studies have shown that vitamin D and its receptor are closely related to diabetic nephropathy and many biological functions of vitamin D are mediated via the transcription regulation of the vitamin D receptor target gene. Many indications exist that suggest that the occurrence of diabetic nephropathy is associated with vitamin D receptor gene genetic polymorphisms.
VDR gene polymorphism in DN
Vitamin D receptor genes are located on chromosome 12 long arm q12-14, with a length of 75 KB containing eight 9 exons and 8 introns and exists as a member of the steroid receptor family. The SNPs connected with DN are mainly located on four variation points including Fok(rs2228570), Bsm(rs1544410) Apa(rs7975232) and Taq(rs731236).
The most investigated VDR gene polymorphism is FokI, which can suppress the first translation initiation site resulting in a peptide lacking three amino acids [37]. The FF genotype of FokI polymorphism had been associated with higher VDR mRNA copy numbers and increased transcriptional activity of VDR [38].
Studies at home and abroad have shown that the FokI allele gene is the initiation codon of the exon and there is statistical significance in the frequency between the case group and control group. Meta-analysis results indicated that there was a significant association between the VDR gene FokI polymorphism and DR susceptibility [39].
Bsm, Apa and Taq are all located at the 3’untranslated region of the gene, which is involved in the regulation of gene expression, especially through the modulation of mRNA stability [40]. It was interesting to note that significant linkage disequilibrium was found among the TaqI, ApaI and BsmI polymorphisms [41]. However, their association with the risk of DN remains controversial.
In summary, the vitamin D receptor and its receptor gene polymorphism are closely related to diabetic nephropathy and its mechanism of action is raising increasing attention. Early intervention in patients with diabetic kidney disease susceptibility is of great significance to prevent the development of diabetic nephropathy. With an increasing number of clinical studies on the vitamin D receptor gene polymorphism, further clues are provided to access the pathogenesis and treatment of diabetic nephropathy.
Conclusion
The genesis of diabetic nephropathy has not yet been elucidated, although hemodynamic changes, high glucose metabolism disorder, inflammatory cytokines and other genetic factors may be involved in the pathogenesis of DN. Once DN develops into the stage of renal failure, treatment is very difficult. Beyond all doubt, the inducing factors vary and are interconnected with each other, combining into a unified identity. The correlation between gene polymorphisms and DN has been taken seriously at home and abroad. With an ever-increasing access to this subject, we can further our understanding of the pathogenesis of DN and broaden the research field, until accomplishing the target of prevention of DN through the creation of a new target drug and accurate prediction of disease. Further study about the genetic mechanism of DN especially T1DN remains a vital task.
References
- Collins AJ, Foley RN, Chavers B (2014) USRenalData system 2013 annual data report. Am J Kidney Dis 63: A7.
- Atkins RC, Zimmet P (2010) World Kidney Day 2010: diabetic kidney disease- act now or pay later. Am J Kidney Dis55: 205–208.
- Mora-Fernández C, Domínguez-Pimentel V, de Fuentes MM, Górriz JL, Martínez-Castelao A, et al. (2014) Diabetic kidney disease: from physiology to therapeutics. J Physiol 592: 3997-4012.
- Ng DP, Placha G, Choo S, Chia KS, Warram JH, et al. (2006) A disease haplotype for advanced nephropathy in type 2 diabetes at the ACE locus. Diabetes 55: 2660-2663.
- Ng MC, Baum L, So WY (2006) Association of lipoprotein lipaseS447X, apolipoprotein E exon 4, and apoC3- 455T>C polymorphisms on the susceptibility to diabetic nephropathy. Clin Genet 70: 20-28.
- Ahn JH, Hong HC, Cho MJ (2012) Effect of Eplerenone, a selective aldosterone blocker,on the development of diabetic nephropathy in Type 2 diabetic rats. Diabetes Metab J 36: 128-135.
- Wang S, Zhang Z, Zhu X, Wu H, Gao H, et al. (2014) Effect of aldosterone and its antagonist on the expression of PAI-1 and TGF-β1 in rat hepatic stellate cells. Int J ClinExp Med7: 4677-4685.
- Ballerman BJ, Zeidel ML, Gunning ME, Brenner BM (1991) Vasoactive peptides and the kidney. In: Brenner BM, Rector FC (eds).The Kidney, 4th edn. Philadelphia: Saunders 510–583.
- Karalliedde J, Maltese G, Hill B, Viberti G, Gnudi L (2013) Effect of Renin-Angiotensin System Blockade on soluble klotho in patients with Type 2 diabetes, systolic hypertension, and albuminuria.Clin J Am SocNephrol8: 1899–1905.
- Kuro-o M (2012) Klotho in health and disease. Curr Opin Nephrol Hypertens 21: 362–368.
- Hu MC, Kuro-o M, Moe OW (2012) Secreted klotho and chronic kidney disease. Adv Exp Med Biol 728: 126–157.
- Hu MC,Kuro-o M,Moe OW (2013) Renal and extrarenal actions of Klotho.SeminNephrol 33: 118-129.
- Asai O, Nakatani K, Tanaka T (2012) Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. KidneyInt81: 539-547.
- Cheng MF, Chen LJ, Cheng JT (2010) Decrease of Klotho in the kidney of streptozotocin-induced diabetic rats. J Biomed Biotechnol 2010: 513853.
- Mitani H, Ishizaka N, Aizawa T (2002) In vivo klotho gene transfer ameliorates angiotensin II- induced renal damageÃÆïÃâüÃâà ½Hypertension 39: 838-843.
- Askari B, Wietecha T, Hudkins KL (2014) Effects of CP-900691, a novel peroxisome proliferator-activated receptor α, agonist on diabetic nephropathy in the BTBR ob/ob mouse. Lab Invest 94: 851-862.
- Lo CS, Liu F, Shi Y (2012) Dual RAS blockade normalizes angiotensin-converting enzyme-2 expression and prevents hypertension and tubular apoptosis in Akita angiotensinogen-transgenic mice. Am J Physiol Renal Physiol302: F840-F852.
- Doi S, Zou Y, Togao O (2011) Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J BiolChem 286: 8655-8665.
- Nusse R, Varmus HE (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31: 99-109.
- Xiao L, Wang M, Yang S (2013) A glimpse of the pathogenetic mechanisms of wnt/beta-catenin signaling in diabetic nephropathy. Biomed Res Int 2013: 987064.
- Zhou L, Li Y, Zhou D, Tan RJ, Liu Y (2013) Loss of klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. JAm SocNephrol 24: 771-785.
- Liu H, Fergusson MM, Castilho RM (2007) Augmented Wntsignaling in a mammalian model of accelerated aging. Science 317: 803-806.
- Ye J (2008) Regulation of PPARgamma function by TNF-alpha. BiochemBiophys Res Commun 374: 405-408.
- Yang J, Zhou Y, Guan Y (2012)PPARγ as a therapeutic target in diabetic nephropathy and other renal diseases. Curr Opin Nephrol Hypertens 21: 97-105.
- Lee EY, Soo Kim S, Lee JS (2014) Soluble α-klotho as a novel biomarker in the early stage of nephropathy in patients with type 2 diabetes. PLoS One 9: e102984.
- Yoon HE, Ghee JY, Piao S (2011) Angiotensin II blockade upregulates the expression of klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrol Dial Transplant26: 800–813.
- Yang H, Wang Q, Li S (2016) MicroRNA-218 promotes high glucose-induced apoptosis in podocytes by targeting heme oxygenase-1. Biochem Biophy Res Commun 471:582–588.
- Bhatt K, Natarajan KM (2016) R: mini-review emerging roles of microRNAs in the pathophysiology of renal diseases. J Am J Physiol Renal Physiol 310: F109-F118.
- He Y, Huang C, Lin X, Li J (2013) MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie 95: 1355–1359.
- Peng H, Zhong M, Zhao W (2013) Urinary miR-29 correlates with albuminuria and carotid intima-media thickness in Type 2 diabetes patients. PLoS ONE 8: e82607.
- Long J, Wang Y, Wang W, Chang BH, Danesh FR (2011) MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem 286: 11837-11848.
- Shiffman D, Pare G, Oberbauer R (2014) A gene variant in CERS2 is associated with rate of increase in albuminuria in patients with diabetes from ONTARGET and TRANSCEND. PLoS ONE 9: e106631.
- Hannun YA, Obeid LM (2011) Many ceramides. J BiolChem 286: 27855–27862.
- Zang L, Fu P, Huang YQ (2012) [Vitamin D deficiency and carotid artery intima-media thickness and coronary calcification in patients with diabetic nephropathy]. Sichuan Da XueXueBao Yi Xue Ban 43: 420–4, 450.
- Basit S (2013) Vitamin D in health and disease: a literature review. Br J Biomed Sci 70: 161–172.
- Heaney RP (2008) Vitamin D in health and disease. Clin J Am SocNephrol 3: 1535–1541.
- Uitterlinden AG, Fang Y, Van Meurs JBJ, Pols HAP, Van Leeuwen JPTM (2004) Genetics and biology of vitamin D receptor polymorphisms. Gene 338: 143–156.
- Ogunkolade BW, Boucher BJ, Prahl JM (2002) Vitamin D receptor (VDR) mRNA and VDR protein levels in relation to vitamin D status, insulin secretory capacity, and VDR genotype in Bangladeshi Asians. Diabetes 51: 2294–2300.
- Zhang Y, Xia W, Lu P, Yuan H (2016) The association between VDR gene polymorphisms and diabetic retinopathy susceptibility: A systematic review and Meta-analysis. BioMed Res Int 2016: 5305282.
- Uitterlinden AG, Fang Y, van Meurs JB, van Leeuwen H, Pols HA (2004) Vitamin D receptor gene polymorphisms in relation to vitamin D related disease states. J. Steroid BiochemMolBiol 89–90:187–193.
- Li L, Wu B, Liu JY, Yang LB (2013) Vitamin D receptor gene polymorphisms and type 2 diabetes: a meta-analysis. Arch Med Res 44: 235–241.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences