m-TOR Inhibitors in the Current Practice
Fakhriya Alalawi, Ajay Sharma and Ahmed Halawa
DOI10.21767/2472-5056.100026
Fakhriya Alalawi1,2*, Ajay Sharma2,3 and Ahmed Halawa2,4
1Department of Nephrology, Dubai Health Authority, UAE
2Faculty of Health and Science, Institute of Learning and Teaching, University of Liverpool, UK
3Royal Liverpool University Hospital, UK
4Sheffield Teaching Hospitals, Sheffield, UK
- *Corresponding Author:
- Fakhriya J Alalawi
Department of Nephrology
Dubai Hospital, Dubai Health Authority, UAE
Tel: 00971509787597/0097402195000
E-mail: fjalalawi08@yahoo.co.uk
Received Date: January 05, 2017; Accepted Date: January 13, 2017; Published Date: January 17, 2017
Citation: Alalawi F, Sharma A, Halawa A (2017) m-TOR Inhibitors in the Current Practice. J Clin Exp Nephrol 1:26. DOI: 10.21767/2472-5056.100026
Copyright: © 2017 Alalawi F, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
for long time, calcineurin-inhibitors (CNIs) was the best broadly used immunosuppressant in organ transplantation; since it had proven its efficacy in the reduction of acute rejection episodes and early graft loss, though their use in the long-term, are associated with chronic allograft nephropathy (CAN), besides it increases the cardiovascular risks such as diabetes, hypertension, and dyslipidemias. Moreover, CNIs in combination with other immunosuppressant's increases the risk of fatal infections and malignancy. Ultimately the introduction of the mammalian focus of rapamycin (mTOR) inhibitors, such as sirolimus and everolimus in 1990s, had changed the face of transplantation and conveyed a great hope to transplant physicians as an innovative class of effective immunosuppressants with a unique mechanism and less nephrotoxic effects (1-6).
In this review article we will try briefly to elicit the clinical indications, advantage and disadvantage of m-TOR inhibitors over other immunosuppressant’s medications and their clinical indications inside and outside the transplant field.
Keywords
m-TOR Inhibitors; Sirolimus; Everolimus; CNI; Chronic Allograft Nephropathy (CAN).
Introduction
Renal transplantation remains the best treatment approach for most patients with end-stage renal disease. It yields superior life quality compared with dialysis therapy.
With the great advances in the medical field and the introduction of new and potent immunosuppressant medications, the incidence of acute rejection episodes had declined markedly over the last decade to 5-20% in the first post-transplant year; yet the graft half-life remained practically unaffected [1,2]. Reasons behind that were attributed to the adverse effects of immunosuppressant's, with allograft loss mainly due to chronic allograft nephropathy (CAN) [1,3].
CAN describe the inevitable steady decrease in renal capacity with time; characteristically renal biopsy shows tubular atrophy, interstitial fibrosis, glomerulosclerosis and vascular occlusive changes [1,3].
Other causes of renal allograft loss beside CAN, are death from cardiovascular diseases, infections or malignancy in patients with a functioning graft, which explains the remaining 50% of graft losses.
Unpredictably, with utilization of strong immunosuppressant's medications; the occurrence of de novo malignancies had expanded rapidly, which was attributed to the direct oncogenic impacts of these medications.
The introduction of m-TORI; sirolimus and everolimus in the field of transplantation had brought a great hope to transplant physicians as they have distinctive method of activity and different adverse effects profile (i.e. lower nephrotoxicity, less hypertension and perhaps a protective probability against neoplastic activities) than CNI therapy [1-6].
Mode of Action of m-TOR Inhibitors
Sirolimus (Rapamune®/rapamycin) is a lipophilic microcyclic lactone antibiotic, segregated from a strain of fungus called Streptomyces hygroscopicus, found at Rapa Nui in 1969 [7,8]. Though it was created as an antifungal drug against candida albicans, aspergillus fumigatus and cryptococcus neoformans, it was found later to have an immunosuppressive and anti-proliferative activities that led to its launch as a major immunosuppressant against transplant rejection [1,5-9].
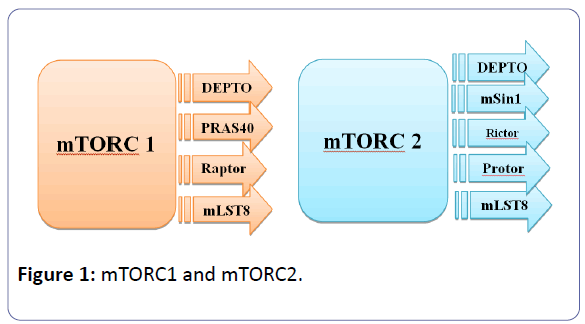
Sirolimus is the prototype of the first generation of mTOR inhibitors and resemble in its structure tacrolimus structure, as it fixes to the immunophilin FK binding protein-12 (FKBP-12), to form an immunophilin complex (catalyst). The objective of this SRL-FKBP-12 complex is the serine-threonine kinase of the phosphatidyl-inositol-3-kinase pathway, which is called mTOR and acts through the co-stimulatory and cytokine-driven pathways, to prevent the signal III pathway (G1 to S transition, interpretation, and cytokine-driven T-cell multiplication) [1,4-10]. The serine/threonine kinase mTOR is a downstream effector of the PI3K/AKT pathway, and forms two distinct multiprotein complexes, mTORC1 and mTORC2 (figure 1). These two complexes have a separate network of protein partners, feedback loops, substrates, and regulators [11].
It appears that growth factors, amino acids, ATP, and oxygen levels regulate mTOR signalling. Several downstream pathways that regulate cell-cycle progression, translation, initiation, transcriptional stress responses, protein stability, and survival of cells are signalling through mTOR [12].
mTORC1 consists of mTOR and two positive regulatory subunits, raptor and mammalian LST8 (mLST8), and two negative regulators, proline-rich AKT substrate 40 (PRAS40) and DEPTOR [13], while mTORC2 consists of mTOR, mLST8, mSin1, protor, rictor, and DEPTOR [14]. mTORC1 is sensitive to rapamycin but mTORC2 is considered to be resistant and is generally insensitive to nutrients and energy signals. mTORC2 is activated by growth factors, and it phosphorylates PKCα, AKT and paxillin, and regulates the activity of the small GTPase, Rac, and Rho related to cell survival, migration and regulation of the actin cytoskeleton. The activity of this complex is regulated by rapamycin, insulin, growth factors, phosphatidic acid, certain amino acids and their derivatives (e.g., l-leucine and β-hydroxy β-methylbutyric acid), mechanical stimuli, and oxidative stress [12].
The mTORC1 signalling cascade is activated by phosphorylated AKT and results in phosphorylation of S6K1, and 4EBP1, which lead to mRNA translation [13].
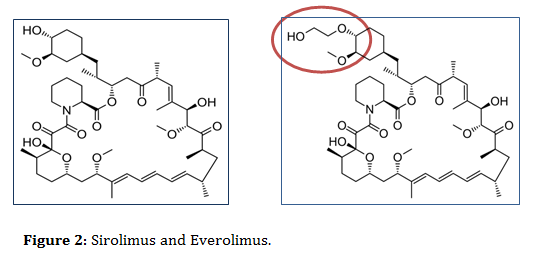
In contrast to SRL, everolimus (EVR/RAD/Certican) is considered as a derivative of sirolimus, structurally looks similar to SLR but with additional extra hydroxyethyl chain substitution at position-40 on the sirolimus molecule, which make it more hydrophilic and bioavailable than sirolimus (figure 2) [1,4,5,7-15].
Similarly EVR/FKBP-12 multiplexes acts directly to mTOR, leading to inhibition of T-cell progression from the G1 to the S stage of cell cycle, thus results in inhibition of IL-2-induced protein synthesis and cellular proliferation and inhibition of IL-4- dependent multiplications of T-and B-cells [1,4,15,16]. Moreover, B-cell activation, proliferation, differentiation into antibody-producing cells, and antibody release are also inhibited [5,6,17]. Indirectly, mTOR inhibitors (sirolimus and RAD) inhibits effector functions of CD4- helper cells and CD8- cytotoxic cells, and activate monocytes, macrophages and other pro-inflammatory leukocytes [5,6,17].
Sirolimus has shown an affinity for FKBP12 that is 2 times greater than tacrolimus and 3.3 times greater than RAD [1,5].
Each of these inhibitors can work synergistically if added to CNIs, although their pharmacokinetic and pharmacodynamic characters can differ between the two drugs owed to the minor differences between their chemical structures.
Pharmacokinetics and Pharmacodynamics
Sirolimus is rapidly absorbed in the intestines, with median T-max (time-of-maximal-concentration) of 0.5 to 2 hours. It is metabolized by both cytochrome P450 IIIA4 (CYP3A4) and P-glycoprotein, which explains the interactions with other drugs and high-fat meals and their metabolites are eradicated through gastrointestinal tract (91%) and kidneys [7,18-21].
The maintenance dose for sirolimus is usually 2 to 5 mg/day, aiming at trough levels of 5 to15 ng/ml. SRL has a very long half-life (nearly 62- 65 hours), so trough level monitoring should be done 5 to 7 days after initiating the medication, which makes once daily dosing probable. In contrast to cyclosporine A, sirolimus trough concentrations are stable over time and strongly correlate with 24-hour exposure (area under the curve [AUC]. There is no significant effect on C-max (maximal concentration) or AUC by sex, age, or ethnic origin [19,20,22].
However, cyclosporine A increases sirolimus bioavailability by 240% when administered simultaneously and by only 80% when administered 4 hours apart. On the other hand, tacrolimus does not have the similar pharmacokinetic interactions with sirolimus as cyclosporine, though (as cyclosporine) it may enhance the adverse effect of sirolimus (including hemolytic uremic syndrome/thrombotic thrombocytopenic purpura/thrombotic microangiopathy (HUS/TTP/TMA) if given concurrently. In a crossover study, simultaneous and separate (by four hours) administration of sirolimus and tacrolimus were compared, and no significant interactions were found in pharmacokinetic parameters, including AUC and C-max, hence combination of sirolimus and tacrolimus can be taken concurrently while administration of cyclosporine should be separated four hours apart from sirolimus [21,23,24]. Moreover, cyclosporine should be administered at lower doses with lower target serum cyclosporine concentrations when given with sirolimus [24].
Similarly, co-administration of diltiazem, verapamil, fluconazole, ketoconazole, anticonvulsants and rifampicin can altered the AUC of sirolimus [5,7,23].
Sirolimus should be taken consistently either with or without food. Grapefruit juice should be avoided since it can unpredictably alter sirolimus pharmacokinetics. Oral tablets of sirolimus are not bioequivalent to the oral solution [23].
Contrary, EVR shows a slightly greater AUC, with half-life of 30hours compared to sirolimus, hence twice-daily dosing is necessary. Equally to sirolimus; it is metabolized by liver and intestinal cytochrome P-450 enzyme CYP-3A, therefore it is affected by drugs and dietary changes.
Pharmacokinetics of EVR are less affected by cyclosporine than sirolimus, therefore it's possible to be administered with cyclosporine or tacrolimus simultaneously [5]. Beyond that, the efficacy of both drugs appears to be similar, as well as their toxicity profiles [7,25-28].
Liver disease significantly increases sirolimus/everolimus bioavailability, reducing its clearance and prolonging its elimination half-life [23].
m-TOR I in Renal Transplantation
There are numerous clinical trials of m-TOR-I since the time it was approved for practice in the renal transplant field; various regimens had appeared aimed at minimizing CNI or eliminating them. Accordingly three CNI-sparing strategies: CNI withdrawal, CNI minimization, and CNI avoidance have been studied [29]; here we summarize the most famous clinical trials used with mTOR inhibitors:
| Trial Name & method | Time of conversion | Results |
|---|---|---|
| SMART: is an observational study, included 132patients followed for 36 month, the aim was to compare early conversion to SRL while watching such effect on eGFR |
Early conversion of CNI to SRL at 10-24 days | • After 36 month, renal function was better in SRL-group (ITT-eGFR at 36month: 60.88 vs 53.72 [CsA] ml/min/1.73 m2, P=0.031), however, many patients had to stop the treatment in the SRL group 59.4% vs 42.3% (CsA). • Survival at 36 months was excellent for both groups (99% [SRL] vs 97% [CsA] and graft; 96% [SRL] vs 94% [CsA]). |
| Rapamune Maintenance Regimen [30] is a prospective, open-labeled, randomized, multicenter trial, included 525patients with aim to compare early conversion to SRL while watching the effect on cGFR over 24 months | Early conversion of CsA to SRL at 3 months | • At 24 months, the differences in patient survival (94.0% vs 95.3%), graft survival (91.2% vs 93.5%), BPAR after randomization (5.1% vs 9.8%) or discontinuations (34% vs 33%) for SRL-CsA-ST vs SRL-ST, respectively, all were not statistically significant • SRL therapy resulted in significant long term improvement in graft survival and BP without increased risk of graft loss or late acute rejection, Calculated GFR (43.8 vs 58.3 mL/min, P<.001). |
| Spare-the Nephron [31] is a prospective, open-label, randomized, multicenter trial, includes 305patients, examined early withdrawal from CNI-based therapy post transplantation while maintaining patients on MMF together with SRL. Patients were followed for a median time of 519 days. | Early withdrawal of CNI at 4-12 weeks | • Patients who were maintained on MMF/CNI for ≤6month and then converted to maintenance immunosuppression with MMF/SRL had greater improvement in mGFR vs patients who remained on MMF/CNI. • The incidence of BPAR was significantly greater with MMF/SRL (12.2%) vs MMF/CNI (4.1%, P=0.02). Graft loss occurred in 3.4% of the MMF/SRL-treated patients and in 8.3% of the MMF/CNI-treated patients (P=0.04). Malignancies were seen less frequent with MMF/SRL. • Withdrawal for adverse events was 34.2% of the MMF/SRL-treated patients and for 24.1% for MMF/CNI-treated patients (P=0.06). |
| CONCEPT [32] is a prospective, open-labeled multicenter trial, included 237patients, evaluated for early conversion from a CsA-regimen to a SRL-regimen, measuring eCrCl (Cockcroft and Gault) at 52weeks | Early conversion at 3 months from CNI to SRL | • CrCl at 52weeks was superior in SRL group (68.9 vs 64.4 mL/min, P=0.017), though patients and grafts survival were statistically insignificant. • The frequency of acute rejection, had occurred mostly following steroids withdrawal, however, although it was numerically higher but statistically insignificant in the SRL group (17% vs 8%, P=0.071). |
| CONVERT [33] 830patients were randomly assigned to continue CNI (n:275) or to be converted from CNI to SRL (n:555), Endpoint were GFR (Nankivell), rates of BPAR, allograft loss, or death at 12-24 months. |
Late conversion from CNI to SRL (6-12 months. | • ITT analysis of the primary endpoint failed to demonstrate any significant benefit of SRL conversion group over CNI-group, however, among SRL converted-group; patients with GFR >40 ml/min/1.73 m2 at baseline were accompanied with superior patient and graft survival, while there was no difference in acute rejection episodes and had lower incidence of neoplasia compared with CNI group, as demonstrated by retrospective analysis of binary outcome for the overall stratum. • Superior renal function was observed in SRL-group that persisted through 12-24 months, particularly those with baseline GFR >40 ml/min/1.73 m2 and UPr/Cr ≤0.11. • An unexpected finding of new onset proteinuria had appeared with an increase in the preexisting proteinuria following conversion to SRL. |
Table 1: Trials of CNI elimination using sirolimus in kidney transplantation.
| Trial Name & method | Time of conversion | Results |
|---|---|---|
| ZEUS [34] is an open-label, multicenter RCT, includes 300patients, followed up for 12 months. | Early conversion of CsA to EVL at 4.5 months | • Everolimus therapy was accompanied with an improvement in GFR compared to cyclosporin (71·8 ml/min per 1·73 m2 vs 61·9 ml/min per 1·73 m2, respectively. • BPAR Rates were greater in the everolimus group than cyclosporin after randomisation (15 [10%] of 154 vs five [3%] of 146; p=0·036), • Early replacement of CNI with everolimus-based therapy had improved renal function at 12 months and preserved efficacy and safety. |
| ASCERTAIN [35] is an open-label, multicenter RCT aimed at CNI elimination or minimization while introducing EVL, patients included (N=394 ITT; 127 CNI elimination vs 144 CNI minimization), all followed for 24 months. | Late conversion (>6 months, mean follow-up 5.6 years) | • No overall renal benefit of EVL was shown, adverse events and discontinuations were more frequently noted in the EVL group, however, Post hoc analyses showed that patients with CrCl >50 mL/min at baseline had considerably greater increase in mGFR after CNI eradication vs controls (variance of 11.4 ml/min/1.73 m2 vs 20.8 ml/min/1.73m2, P=017). |
| CENTRAL [36] is an open-label, multicenter RCT, included 202pateints, followed-up for 36 months. | Early conversion of CsA to EVL at 7weeks | • Conversion from CsA to EVL at 7th weeks was accompanied with significant enhancement in mGFR at 36 months vs CsA-treated patients, although drug withdrawals and BPAR episodes were more common • There was no overall advantage in an intent-to-treat population. |
Table 2: Trials of CNI elimination using everolimus in kidney transplantation. Till date there is no head to head trial comparing everolimus to sirolimus in renal transplant recipients, and therefore there is no evidence for superiority of either drug on the other.
Recommendations for Conversion to m- Tor Inhibitors in Renal Transplantation
There is no special protocol for conversion to m-TOR I in renal transplant, however, proteinuria, frequency of rejection episodes that precedes conversion, higher chronic Banff score and high score of vascular intimal thickening were connected with non-responder in a univariate investigation [19]. Diekmann had proposed conversion for CAN at creatinine less than 2.5mg /dl and proteinuria beneath 800 mg/day [22].
Current literature had suggested the likely beneficial evidences for conversion to SRL-based regimen if there are: [1,4,29]
• CNI- related symptoms, for example, nephrotoxicity, arterial hypertension and diabetes mellitus.
• Early chronic transplant dysfunction, however, GFR should be more than 40 mL/min with normal urinary protein excretion.
• Possibility of tumor recurrence/occurrence with the administration of CNI.
On the other hands; the possible contraindications for conversion to SRL-based regimen are:
• Hyperlipidemia with serum cholesterol >300 mg/dl and/or serum triglycerides >400 mg/dl, despite using lipid-lowering agents.
• Advanced renal failure with elevated serum creatinine that exceeds 4 mg/dl.
• Glomerular damage with proteinuria >1 g/day at baseline.
The optimum time for early conversion is about 3 months post-transplant.
Notably; The administration of SRL in high-risk group, like those with diabetes mellitus, body mass index >30 kg/m2, major re-operations or delayed graft function, should not occur until four to six weeks post transplantation, after the wound had healed and serum creatinine level stabilized at 2.5 mg/dl or below.
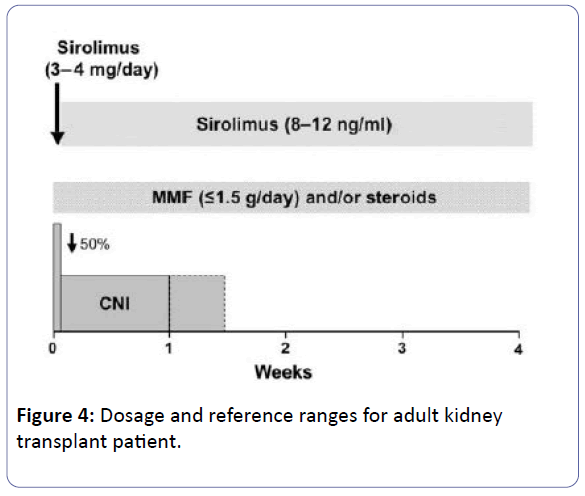
The dosage of MMF/AZA must be decreased by 50% at the beginning of SRL therapy while steroid treatment might be continued [1].
The CNI dosage must simultaneously decreased by 50% and it can be ceased totally when the SRL trough level reach 5–10 ng/mL. Further trough level measurements are suggested weekly after each extra dose adjustment [1].
Additional trough level estimations are suggested for patients with liver impairment or when using particular substances that might hinder CYP3A4 and/or P-gp metabolism.
Two SRL conversions protocols can be used;
• Abrupt stop of CNI therapy with a single loading dose of sirolimus of 10mg followed by 5 mg/day in African- Americans or a loading dose of 8 mg followed by 4 mg/day in Caucasians [37].
• To introduce sirolimus at a dose of 3-4 mg/day with a synchronous decrease of CNI dosage to 50%. Once target level had achieved (8-12ng/ml), then CNI can be withdrawn totally, commonly in 7-10 days [38,39].
Note: Mycophenolate dose should be ≤ 1.5 g/day [40].
The initial oral sirolimus maintenance dose is 2 to 5 mg daily. Subsequent doses are based on the attainment of therapeutic trough concentrations [1,23].
| ≤ 3 months | >3 months | |
|---|---|---|
| (When sirolimus is used with tacrolimus or cyclosporine +/- MMF/mycophenolic acid and steroids): | 8-12 | 5-10 |
| (When sirolimus is used as a single agent +/- steroids) | 10-12 | 8-10 |
Table 3: Sirolimus Trough Concentration (ng/mL).
For everolimus, an initial dose regimen of 0.75 mg twice daily concurrently with ciclosporin is recommended for the general kidney and heart transplant population, while a dose of 1.0 mg twice daily in co-administration with tacrolimus is recommended for the hepatic transplant population. Subsequent doses are tailored based on blood concentrations level, initial adjustments can be made at 4-5 day intervals after initiating therapy. Recommended everolimus range to be 3 to 8 ng/mL for renal and liver transplant patients [8,23,26].
| Period | Month 1 | Months 2-3 | Months 4-5 | Months 6-12 |
|---|---|---|---|---|
| Target cyclosporin Co (ng/ml) | 100-200 | 75-150 | 50-100 | 25-50 |
| Target tacrolimus (ng/ml) | 3-7 | 3-7 | 3-7 | 3-7 |
Table 4: suggested target CNI trough concentration windows with m-TOR I therapy in renal transplant recipients. C0 levels monitoring is reliable measure with mTORI therapy [41].
Clinical Advantages of mTOR-inhibitors Therapy over Other Immunosuppressants
Antitumoral activity
Immunosuppressants in conjunction with other oncogenic stimuli, such as viruses and radiation can be a major risk for development of certain cancers like Kaposi sarcoma, lymphoma and skin cancers, which is considered the leading reasons for morbidity and death after solid organ transplantation. There is, however, persuading evidence that avoiding CNIs and uses of mTOR-inhibitors can convey advantages with respect to the progression of malignancy [2,19,25,42,43], this was proven on several studies, e.g. patients in CONVERT study who were converted to sirolimus; had a diminishment in the rate of malignancies compared to the individuals who stayed on CNI at 12 and 24 months (3% vs 10%) [1,4,32,33], similarly Campistol JM et al, had reported successful recession of Kaposi’s sarcoma and PTLD with no alteration in renal function after substitution of CNI with mTORIs [44].
mTOR-I has cytostatic effect without cytotoxic activities, so the clinical outcome is stabilization of the tumor cells rather than progression.
The mechanism by which m-TOR-I can incite this anti-tumor activity is related to up-regulation of adhesion molecules and to a change to less aggressive phenotype of tumoral cells, as appeared with Luan et-al. who found that SRL could modify tumor cells from aggressive phenotypes like spindle- or dome-shaped cells to a less aggressive cuboidal form in renal cell carcinoma [45].
Moreover, m-TORI can block growth-factor-stimulating cell proliferation of haematopoietic and non-haematopoietic cells (CNS, hepatocytes, renal, melanocytic, osteoblastic, myogenic, fibroblasts and endothelial cells) by creating a complex with the intracellular immunophilin FKBP-12. Furthermore it inhibits proliferation of T and B cells altered by HTLV-1 and EBV [1,15].
In the pre-clinical models; m-TORI had exhibited growth inhibition of tumoral cells by increasing expression of E-cadherin and p27-kip1, also it decrease cyclin-d1 expression, causing arrest in cellular progression from G1 to S-phase [1,4,6,7,15]. Recent reports demonstrated that sirolimus inhibits UV-B activation of several metalloproteinases that can enhance tumor development and premature skin aging [34].
Lower rate of viral disease after transplantation
It was observed that the rate of viral infections (particularly cytomegalovirus and BK virus infections) are much lower in patients taking mTOR-inhibitor-based regimens than other immunosuppressant, which is true for both sirolimus and everolimus and in all recipients of kidney and heart grafts weather anti-viral prophylaxis were given or not [2].
Everolimus showed a profound inhibitory effect on human Epstein-Barr virus and lymphoblastoid B-cell lines in vitro [5].
Effect of mTOR inhibitors on cardiovascular disease
Cardiovascular disease is known to have a great influences on morbidity and mortality for kidney transplant recipients, risk factors (such as hypertension, left ventricular hyperplasia, hyperlipidemia and Post-transplant diabetes mellitus (PTDM)) has been linked greatly with CNI use [2,4], and by replacing/ minimizing them with mTOR-I we can reduce such risk factors.
Additionally, conversion from CNI-therapy to mTORi-based regimen had improved blood pressure significantly as shown in the RMR study and may achieve regression of left ventricular hyperplasia [39].
Although hyperlipidemia is considered to be a major concern in patients converted to mTOR-inhibitors, with highly elevated cholesterol and triglycerides levels, with subsequent increased use of lipid-lowering agents [2]; yet the long-term follow-up in most studies does not suggest an increased association between mTOR-I’s and cardiac events. Indeed, pre-clinical studies had suggested a beneficial effect of mTORIs on atherosclerosis with favorable effects were found in human cardiac transplantation, in which SRL has been shown to reduce the progression of allograft vasculopathy [4].
Experimental studies have shown that SRL could be effective in reducing aortic atherosclerosis in apo-E-deficient mice independent of lipid levels [2,4].
Clinical Indications of m-TOR I in Non- Transplant Patients
Anticancer therapies
Tumors may occur due to dysregulation of mTOR signals and can confer higher susceptibility to mTOR inhibitions.
Deregulations of multiple elements of the mTOR pathway, like PI3K amplification/mutation, PTEN loss of function/mutation, AKT mutations/amplifications, and S6K1, 4EBP1, and eIF4E overexpression have been related to many types of cancers. Both eIF4E and S6K1 are included in cellular transformation and their overexpression has been linked to poor cancer prognosis [46,47]. Therefore, mTOR is an interesting therapeutic target for treating multiple cancers alone or in combination with inhibitors of other pathways.
Currently m-TOR-I (rapamycin and several rapalogs, including temsirolimus, everolimus, and ridaforolimus which are the first generation mTOR inhibitors) are being widely investigated outside the transplant field for their anti-proliferative properties on certain kinds of tumors, including glioma, non-small cell lung cancer, breast cancer, rhabdomyosarcoma, B- and T-cell lymphoma, multiple myeloma, colonic carcinoma, pancreatic cancer, hepatocellular carcinoma, ovarian cancer, endometrial carcinoma, renal cell cancer, prostatic cancer, bladder cell carcinoma and melanoma, however, their use remained limited within experimental field [1,48]. Probably this is related to the fact that rapalogs are primarily cytostatic, and therefore effective as disease stabilizers rather than for regression, hence the response rate in solid tumors where rapalogs have been used as a single-agent therapy have been modest [49-52]. Another reason for the limited success is the presence of feedback loop between mTORC1 and AKT in certain tumor cells. It seems that mTORC1 inhibition by rapalogs fails to suppress a negative feedback loop that results in phosphorylation and activation of AKT [48,53-55]. These limitations have led to the development of the second generation of mTOR inhibitors known as ATP-competitive mTOR kinase inhibitors [55].
Coronary stent coating
The antiproliferative effect of m-TOR I has also been used in conjunction with coronary stents to prevent restenosis in coronary arteries following balloon angioplasty. The sirolimus or everolimus is formulated in a polymer coating that affords controlled release through the healing period following coronary intervention.
Several large clinical studies have demonstrated lower restenosis rates in patients treated with sirolimus-eluting stents when compared to bare-metal stents, resulting in fewer repeated procedures [56].
Moreover, sirolimus-eluting stents (SES) were found to have superior results compared to everolimus-eluting stents (EES), which were associated with greater angiographic in-segment late loss and higher rates of in-segment restenosis compared with SES implantation, though clinical outcomes were both excellent and not statistically significant [57].
Lymphangioleiomyomatosis
(LAM) is a rare, progressive and systemic disease, predominantly affects women of childbearing age and typically resulting from proliferation in the lung, kidney, and axial lymphatics of abnormal smooth muscle–like cells (LAM cells) that exhibit features of neoplasia and neural crest origin. Cystic destruction of the lung with progressive pulmonary dysfunction and presence of abdominal tumors (eg, angiomyolipomas [AML], lymphangioleiomyomas) characterizes the disease. It usually occurs as a feature of an inherited tuberous sclerosis complex (TSC-LAM), with mutations of the tuberous sclerosis complex gene (TSC2), though sporadic forms of the disease (S-LAM) are present. Loss of TSC2 gene function activates the mTOR signaling pathway, resulting in the release of lymphangiogenic growth factors and this can be blocked with m-TOR I.
Sirolimus was approved by the Food and Drug Administration for their use in LAM in May 2015, based on the results of Multicenter International LAM Efficacy and Safety of Sirolimus (MILES) Trial. The MILES data supports the use of sirolimus in patients who have abnormal and rapidly declining lung function (i.e. FEV1<70% predicted). Sirolimus also appears to be effective for the treatment of chylous effusions. The benefits of sirolimus only persist while treatment continues, so the safety of long term therapy must be addressed in further studies [58-60].
Sirolimus is the only and first drug therapy approved by the FDA in the management of lymphangioleiomyomatosis (LAM).
Neurological Indications
Tuberous sclerosis complex and epilepsy
Sirolimus/Everolimus shows a promising role in treating tuberous sclerosis complex (TSC), elicited on several studies that conclusively linked mTOR inhibitors to remission in TSC tumors, specifically subependymal giant-cell astrocytomas in children and renal angiomyolipomas in adults, and pulmonary lymphangioleiomyomatosis [61-64]. Further trials are ongoing, till then their uses will remain off-label [46], moreover, tumors were often re-grow when the treatment stopped.
Topical sirolimus therapy (applied in different formulations such as ointment, gel, solutions, and creams, ranging from 0.003 to 1% concentrations) was used for treatment of facial angiofibromas with positive outcomes results reported in sixteen separate studies involved a total of 84 patients and improvement was observed in 94% of subjects, especially if treatment began during the early stages of the disease [47,65].
In addition to the known role of the mTOR in tumorigenesis and the associated utility of mTOR inhibitors for treating tumors in TSC, the importance of mTOR in the common, disabling neurological symptoms of TSC, in particular epilepsy, autism, and cognitive deficits, is not well established and appear to be unrelated to tumor growth per se, but probably linked to other cellular and molecular abnormalities, such as aberrant circuit formation and dysregulated neurotransmitter receptors or ion channels. However, whether mTOR inhibitors represent a rational treatment for seizures and neuropsychiatric symptoms in TSC patients, this has not been prompted yet in the clinical practice [63]. Furthermore, there is increasing interest as to whether the mTOR pathway may be involved in other types of epilepsy (other than genetic epilepsies), such as epilepsy following acquired brain injury. There is some evidence that mTOR inhibitors can inhibit existing seizures or can prevent epilepsy in some animal models of acquired epilepsy, though other studies have found negative results [65-69].
Non–TSCrelated brain tumors
In addition to SEGAs in TSC, the mTOR pathway has been implicated in the pathophysiology of other brain tumors unrelated to TSC, particularly other types of gliomas [65]. The mechanism of the antitumor effects of rapamycin against gliomas is still being investigated, but may include direct cytotoxic and antiproliferative effects, inhibition of vascular endothelial growth factor and angiogenesis, decreased invasive propensity, and increased sensitivity to radiation [70].
Neurodegenerative diseases
M-TOR pathway has been implicated in a number of neurological conditions including structural brain disorders, inherited neurocognitive disorders such as fragile X syndrome, autism spectrum disorder, and classical progressive neurodegenerative diseases such as Alzheimer disease, Parkinson’s and Huntington disease which characterized by accumulation of abnormal toxic proteins and associated neuronal death.
Since mTOR pathway is involved in regulation of cell death and survival mechanisms, it makes sense that mTOR has also been implicated in the pathophysiology of these neurodegenerative disorders. In particular, mTOR signaling may modulate the mechanisms of apoptotic cell death. Furthermore, mTOR pathway normally inhibits autophagic mechanisms, which help degrade and clear aggregated or accumulated proteins. Thus, mTOR inhibitors may represent a rational therapy and protective gents for this group of diseases by inducing autophagy or by directly regulating neuronal death mechanisms. However their use is limited currently to animal’s models. Moreover, significant side effects that may occur with mTOR inhibitors, including chronic immunosuppression and associated opportunistic infections, make this prospect of longterm treatment less attractive [65].
Muscular dystrophy in mice
Duchenne muscular dystrophy progresses rapidly in males to severe impairment of muscle function and death in the second or third decade of life. Bibee et al had demonstrate an alternative approaches that rescue defective autophagy in mdx mice, a model of Duchenne muscular dystrophy, with the use of rapamycin-loaded nanoparticles induce a reproducible increase in both skeletal muscle strength and cardiac contractile performance that is not achievable with conventional oral rapamycin, even in pharmacological doses. This increase in physical performance occurs in both young and adult mice, and, surprisingly, even in aged wild-type mice. Although the exact mechanism responsible for rapamycin's entry into muscle tissue remains to be clarified, the effect on autophagy is clear and seems dependent on NP-linked depot delivery, because oral therapy is ineffective at the doses that were administered [71,72].
Progeria (Hutchinson–Gilford Progeria Syndrome (HGPS))
Progeria or aging disease is an extremely rare genetic disorder in which symptoms resembling aspects of aging and manifested at a very early age leading to an extremely compromised cell-damage repair capacity and typically resulting in death in the early teenage years due to causes which are generally associated with old age such as heart disease or stroke.
A recent discovery that rapamycin suppresses a pro-senescent phenotype in progeric cells in addition to clearance of progerin. Rapamycin in theory would suppress geroconversion downstream of progerin. Furthermore, rapamycin prevents atherosclerosis in animal models of accelerated atherosclerosis and accelerated atherosclerosis is one of the main symptoms of progeria leading to death [73].
Others genetic diseases
Tuberous sclerosis, Peutz-Jeghers syndrome, Cowden syndrome, Bannayan-Riley-Ruvalcaba syndrome, Lhermitte- Duclos disease, Proteus syndrome, von Hippel-Lindau disease, Neurofibromatosis type1 are all caused by mutations in the mTOR pathway component genes TSC1, TSC2, LKB1, PTEN, VHL, and NF1 respectively, hence addition of m-TOR I can play a promising role in such diseases [74].
Systemic lupus erythematosus in murine lupus models and human
Sirolimus decreases the disease activity and prednisone requirement in systemic lupus erythematosus (SLE) patients who are intolerant or resistant to immunosuppressant medications.
Sirolimus acts through blocking the activation of its molecular target, the mechanistic target of rapamycin complex 1 (mTORC1). The mTOR pathway is involved in many aspects of T cell differentiation and function. The activation of mTORC1is associated with suppression of mTORC2, results in the expansion of pro-inflammatory CD4-CD8- double-negative (DN) T lymphocytes. These DN-T cells produce inflammatory cytokines, interleukin-4 (IL-4) and interleukin-17, and they exhibit predisposition to pro-inflammatory cell death through necrosis. Moreover, Increased IL-4 production is responsible for activation of autoantibody-producing B lymphocytes in SLE. Rapamycin treatment in vivo blocked the IL-4 production and necrosis of DN T cells, increases the expression of FOXP3 in CD25(+)/CD4(+) T cells, and expanded CD25(+)/CD19(+) B cells. These results identify mTOR activation to be a trigger of IL-4 production and necrotic death of DN T cells in patients with SLE.
It is possible that rapamycin treatment resulted in recovery of IL-2 production along with normalization of calcium signaling, creating a cytokine milieu more favorable to regulatory T cell function. Rapamycin also has been demonstrated to promote an anti-inflammatory environment by affecting the behavior of dendritic cells. M-TOR inhibition leads to complementary changes in myeloid dendritic cells and T cells leading to the production of regulatory T cells. This regulatory T cells together with CD4+CD25-cells increased STAT3 and STAT5 phosphorylation and enhance the differentiation of CD8+ T cells into memory cells in mice inoculated with lymphocytic choriomeningitis virus. Prospective clinical trial in SLE patients with sirolimus is ongoing [74-76].
Scleroderma
m-TOR can plays a role in fibrotic diseases and autoimmunity, and blockade of the mTORC pathway through blocking the pro-fibrotic effects of TGF-β can play a part in the treatment of scleroderma (or systemic sclerosis), however, this approach is currently under investigation [77].
Glycogen storage disease (GSD)
In a trial done in a canine model of glycogen storage disease (GSD) IIIa, had suggested that rapamycin can inhibit mTORC1, so that the phosphorylation of GS (glycogen synthase) can be increased in skeletal muscle and glycogen content in primary muscle cells can be reduced significantly in patients with GSD IIIa through suppressing the expression of glycogen synthase and glucose transporter 1, therefore rapamycin had prevented effectively progression of liver fibrosis in GSD IIIa dogs, consistent with markedly inhibiting the transition of hepatic stellate cells into myofibroblasts, the central event in the process of liver fibrosis. This discovery represents a potential novel therapeutic approach for glycogen storage diseases (for GSD III) [78-79].
ADPKD
Sirolimus has been assessed as a therapeutic option for autosomal-dominant polycystic kidney disease (ADPKD). Different case reports demonstrated sirolimus as a key role in reducing kidney volume in patients with early-stage ADPKD and delaying decline in kidney function compared with those receiving standard care. However, the current clinical trials are limited with confounding results [79-81].
Familial cardiac hypertrophy and Wolff-Parkinson- White syndrome
An essential mediator of cardiac myocyte enlargement is protein synthesis, which is controlled at the levels of both translation-initiation and elongation. Eukaryotic elongation factor-2 (eEF2) mediates the translocation step of peptide-chain elongation and is inhibited through phosphorylation by eEF2 kinase. In addition, p70S6 kinase can regulate protein synthesis by phosphorylating eEF2 kinase or via phosphorylation of ribosomal protein S6. Phosphorylation of eEF2 kinase is also controlled by AMP-activated protein kinase (AMPK), a key regulator of cellular energy homeostasis. Dependent on AMPK-priming phosphorylation of tuberin, GSK3β phosphorylates tuberin and triggers activation of its potential to inhibit mTOR. AMPK γ2, an important regulatory subunit of AMPK, is encoded by the gene PRKAG2. Mutations in PRKAG2 are responsible for familial cardiac hypertrophy and Wolff-Parkinson-White syndrome. The knowledge that an important hallmark of cardiac hypertrophy (a major risk for cardiac morbidity and mortality) is hyperactivation of the PI3K-mTOR cascade, initiated a discussion whether inhibition of the mTOR pathway could be beneficial in treating such disorders [79,82,83].
The mTOR pathway in obesity and type 2 diabetes
Recent studies had linked insulin resistance in type 2 diabetes mellitus and obesity to mTOR Complex1 pathway since it is controlled through nutrient-hormonal signaling network.
Insulin resistance can be regulated by mTORC1 activation of p70S6K through the negative feedback loop. p70S6K has been demonstrated to phosphorylate IRS1 on multiple inhibitory sites promoting its degradation. Inhibition of IRS protein function will desensitize cells to insulin. Phosphorylation of IRS1, which is known to antagonize IRS signaling, is elevated in animal models of obesity and in muscle from type 2 diabetic patients. Insulin resistance is the hallmark for both, obesity and type 2 diabetes [79,84,85].
In HIV
In vitro and in vivo studies suggest sirolimus to possess anti- HIV properties that may qualify it as a potential new anti-HIV drug. Sirolimus inhibits HIV replication through different mechanisms, including down-regulation of the co-receptor CCR5 and induction of autophagy. In addition sirolimus synergistically enhances the anti-HIV activity of entry inhibitors such as vicriviroc, aplaviroc and enfuvirtide in vitro.
Additionally, a prospective nonrandomized trial of HIV patient series receiving RAPA monotherapy after liver transplantation indicated significantly better control of HIV and hepatitis C virus (HCV) replication among patients taking RAPA monotherapy [86].
Anti-Aging properties
Rapamycin was first shown to extend lifespan in eukaryotes in 2006 by Powers et al. who showed a dose-responsive effect of rapamycin on lifespan extension in yeast cells. Based on this and other researchers, in 2009, Harrison De et al had demonstrated a significant increase in the lifespans of genetically heterogeneous mice fed with rapamycin with increase in median survival by 14% for females and 9% for males, and he attributed this to retarding ageing mechanisms or probably to delaying death from cancer. Life span lengthening was confirmed on subsequent studies, and is now being tested in the marmoset monkey and dogs [87-91].
It is hypothesized that dietary regimes such as caloric restriction and methionine restriction, causes lifespan extension by decreasing mTOR activity and it is believed that this is achieved by limiting the essential amino acid leucine and methionine, which are potent activators of mTOR. The administration of leucine into the rat brain has been shown to decrease food intake and body weight via activation of the mTOR pathway [61,62,87,92].
Moreover, and based on the free radical theory of aging, reactive oxygen species cause damage of mitochondrial proteins and decreases ATP production. Subsequently, via ATP sensitive AMPK, the mTOR pathway is inhibited and ATP consuming protein synthesis is down-regulated, since mTORC1 initiates a phosphorylation cascade activating the ribosome. Hence, the proportion of damaged proteins is enhanced. Additionally, disruption of mTORC1 directly inhibits mitochondrial respiration. These positive feedbacks on the aging process are counteracted by protective mechanisms: decreased mTOR activity (among other factors) upregulates glycolysis and removal of dysfunctional cellular components via autophagy [64,93,94].
However, this remains within experimental field and it is not known whether rapamycin will have similar lifespan-lengthening effects in humans.
Side Effects of m-TOR Inhibitors
Majority of m-TORI clinical trials have been done in renal transplant patients using SRL, However, it seems reasonable that EVR can induce similar adverse effects. Apparently that time, drug dosage and high drug trough levels might have a primary role in the development of drug-related adverse effects and clinical complications.
M-TOR-inhibitors have considerable adverse effects that can limit its use in some patients. Nearly 30-50% of patients on SRL therapy have to discontinue the therapy due to these related adverse effects [1,17].
Reported Side Effects of m-TORI Includes
• Hypertension in 8 -58% of cases [3,15].
• Fever (23% to 34%), chest pain, headache, insomnia, fatigue, Arthralgia, alterations in taste, and asthenia are common side effects and can be managed by reducing the drug dose [1,3,15].
Hyperlipidemia
Hyperlipidemia was reported in 8% to 57%,
M-TORI increases high-density lipoproteins (HDL), low density lipoproteins (LDL), cholesterol, and triglycerides in approximately 40 to 75% of patients receiving this therapy, it affects the up-regulation of adipocyte fatty acid-binding protein (aP2) expressed in monocytes and macrophages which plays a key role in the increased accumulation of triglycerides [3,15].
Hyperlipidemia is the principle risk factor for post-transplant cardiovascular-related morbidity and mortality with a yearly hazard appraisal of 50-fold more prominent for renal transplant recipients than for the general population, that is responsible for over one-third of all deaths, however, some authors (as mentioned earlier) claims that the long-term follow-up in patients getting m-TORI does not propose an expanded risk for cardiac events. Indeed few had suggested further beneficial benefits of mTORIs on atherosclerosis.
Joannid`es et al. have demonstrated that SRL-based regimen reduces aortic stiffness, plasma endothelin-1, and oxidative stress in renal recipients proposing a defensive impact on the arterial wall with reduction in the total cardiovascular risk, However, extra studies are necessary to evaluate long-term cardiovascular effects of mTOR-I therapy in renal transplant patients [4,15,42].
Hyperlipidemia with m-TORI is reversible and dose dependent, as shown in Morrisett et al. study; where he observed that cholesterol and triglyceride levels increases 2–4 weeks after initiation of therapy, and reverted to near-baseline levels within 8 weeks after discontinuation of treatment [3,15]. Hyperlipidemia in m-TORI-based regimen can be treated with statins therapy alone or in combination with a second line agent.
Gastrointestinal Effects
Reported in approximately 15–20%, and include stomatitis, abdominal pain, anorexia, nausea, vomiting, diarrhea, constipation and abnormal LFT [42].
Mucositis and oral ulcerations are the commonest side effects reported with mTOR-I therapy, occurs probably secondary to the direct toxic effect of these drugs on oral and nasal mucous membranes.
Mouth ulcers typically are transient and emerge soon after administration of SRL. It could be treated with topical steroid, iodine, or topical analgesic. If symptoms persisted, mTOR-I ought to be ended and possibly restarted at a lower dosage after resolution of symptoms.
Gastrointestinal leukocytoclastic vasculitis is a rare complication reported in a few cases with use of SRL. It is characterized by diffuse mucosal thickening of the antrum, duodenum, and jejunum. Drug discontinuation is necessary if occurs [1,3,15,29].
Increased Infection Risk
Reported in 2-60% in various literatures, occurs early in the post-conversion period and could be attributed to the high loading dosages [1,3,4,15]. Severe bacterial infections can occur in m-TOR-I group, secondary to inhibition of interleukin-12- induced proliferation of activated T lymphocytes and IFN creation of the lymphocytes. Both cytokines are known to be critical in the defensive insusceptibility to intracellular bacteria (e.g., mycobacterium).
Though bacterial infection is common with m-TORI, studies had suggested a protective effects against viral infections particularly CMV infection and possibly BK infection, which could be related to drug inhibitory effects on viral replication [15].
Hematological Effects
Bone marrow toxicity often occurs in patients treated with mTOR-inhibiters, such patients develop anemia, leukopenia, and thrombocytopenia.
Anemia (13-58%), can occurs as early of schedule as 1-month in the SRL-post-conversion group, thereafter, hemoglobin levels typically settled, but at the detriment of incessant utilization of Erythropoietin in around 50% of patients [3,4]. Similarly EVR therapy has been associated with anemia as well [15].
Various mechanisms have been anticipated for mTORI induced anemia, one mechanism proposed the anti-proliferative effect of the drug on bone marrow progenitor cells and possible direct impact on iron homeostasis, other mechanism suggested that mTOR inhibition is responsible for blocking S6 kinase action (S6 kinase assumes a part in mRNA interpretation in the cell), and consequently it hinder cell replication in erythroid cell lines and alter erythroid cell development.
Additionally Thaunat et al. had reported in 2005 a close relationship between chronic inflammatory status and mTOR-I-related anemia. Equally, others reported low serum iron levels and micro-inflammation in patients got converted from CNI to EVR. An alternative hypothesis of the myelosuppressive influence of SRL is due to the inhibition of the signal transduction via the gp-130 [beta] chain shared by a variety of cytokine receptors, including interleukin-11, granulocyte colony stimulation factor, and erythropoietin, which stimulate the production of platelets, leukocytes, and erythrocytes, respectively [3,15].
Leukopenia was noticed in 5-39% with both SRL and EVR, necessitating the use of granulocyte colony-stimulating factors in few patients [3-5,15].
Significant thrombocytopenia was reported in 10%-20% of renal transplant recipients and thought to be dose dependent. Thrombocytopenia is reversible within 2 weeks after discontinuation of the drug; most of those require dose reduction and few needs temporary drug withdrawal. No patient requires permanent discontinuation of therapy [5,15].
TTP/TMA/HUS:
m-TOR I may increase the risk of CNI-induced haemolytic uraemic syndrome/ thrombotic thrombocytopenic purpura/ thrombotic microangiopathy if used in combination with CNI [26].
Respiratory Symptoms (2-11%)
Several varieties of pulmonary injury have been reported in the literatures, including lymphocytic interstitial pneumonitis, lymphocytic alveolitis, bronchiolitis obliterans with organizing pneumonia, focal pulmonary fibrosis, dyspnea and cough.
A standout amongst the most serious symptoms is the development of SRL- induced interstitial pneumonitis. This is usually dose dependent, with an onset of symptoms between 1 to 51 months after the initiation of SRL/EVR therapy. Diffuse alveolar hemorrhage has been accounted too.
The pathogenic mechanism of pulmonary toxicity is still unclear. A cell-mediated autoimmune response was suggested. Lung biopsy had shown several histological features such as intra-alveolar non-necrotizing epitheloid granuloma, lymphocytic interstitial inflammation, and focal pattern of organizing pneumonia [3,4,15,29].
Post-Transplantation Diabetes (3-33%)
To date, just a small number of clinical studies had claimed that m-TORI can cause PTDM, the mechanisms are not clearly understood, though, a few speculations were made: [15,42]
• SRL can induce ectopic triglyceride deposition prompting insulin resistance.
• Impairment of insulin-mediated suppression of hepatic glucose generation.
• Direct toxic impact on pancreatic cells.
• AKT activation with impairment of insulin receptor substrate signaling.
• Another interesting hypothesis is that mTOR is involved in insulin signaling, and its inhibition may impede insulin related gene transcription and expression, including glucose transporters, leading to inhibition of GLUT1mRNA increase, resulting in failure of insulin to stimulate glucose uptake.
• Similarly, mTOR is an inducer of ribosomal S6 kinase (S6K), and SRL blocks S6K activation or induces S6K inactivation through inhibition of T389 phosphorylation interfering with the transcript of insulin. This action may impact blood sugar levels.
Wound Complications
in up to 20-50% of patients, which is significantly higher compared with other immunosuppressive drugs. These include wound dehiscence, lymphocele development, delayed wound healing, incisional hernias, and infections.
Lymphocele formation has an incidence that varies according to different publications, e.g. in (Langer and Kahanshows) study it had reached up to 38% of patients [3,15].
Wound complications are likely identified with SRL capacity to debilitate signal transduction of fibroblast and endothelial growth factors.
Risk factors for wound complications includes older age recipients, obesity, Caucasian race, diabetics, corticosteroid utilization, and higher dose of SRL at early post-transplant period.
Approaches to diminish wound complications consist of avoidance of loading doses, and modification of surgical techniques, for example, suction drain position, usage of non-absorbable sutures while closing, and perhaps delay the introduction of the drug for several weeks particularly for the high risk group (patients with a BMI > 30, Caucasian, and elderly) [1,3,4,15,29].
Lymphedema
Lymphedema is a relatively unusual adverse consequence of mTOR-I treatment. The underlying mechanism is not totally clarified.
Aboujaoude et al. have hypothesized that the lymphedema could be connected with increased lymph flow along with lymphatic interruption in the affected extremities secondary to the surgical procedures, in addition to the well-identified effect of SRL in increasing vascular permeability and vasodilation [95].
Moreover, Huber et al. had demonstrated an anti-lymphangiogenic activity of mTOR-I, which is not restricted to a specific mTOR-I, rather it is a general phenomenon of the whole class.
Before establishing the diagnosis of lymphatic disease caused by mTOR-I, it is essential to rule out other causes such as neoplasia, infection, and venous obstruction. Unfortunately, reduction or discontinuation of the drug therapy is the only treatment for such patients [4,15].
Cutaneous Adverse Effects
This includes lymphedema (described above), acne, epistaxis, vasculitis and nail disorders [1,3,15]:
• Acne, folliculitis: Acne is reported in 15% to 25% in recipients treated with SRL. Predominantly in male patients suggesting hormonal etiology.
• Chronic peripheral edema (8% to 64%), affecting primary lower limbs.
• Angioedema (acute subcutaneous edema), found in 15% of patients, developed within few hours and disappeared in less than 4 days. They were non-pruritic, non-erythematous, and localized mainly on the face, with oral cavity involvement.
• Nail involvement: includes fragile and thin nails, longitudinal ridging, distal onycholysis, and erythema.
• Skin and scalp hair abnormalities include mild alopecia or hypertrichosis of the face.
These adverse events were not serious in most cases; however, in 12% of patients it was necessary to withdraw the therapy [1,3,15].
Gonadal Effects
There are several publications highlighted the effects of m- TORI particularly SRL on male gonads, these studies have focused on sex hormones production, erectile function, and fertility.
It was found that SRL therapy had significantly lowered testosterone levels while it had increases the gonadotrophic hormones (FSH and LH). Additionally, a substantial reduction of total sperm quantity was noticed.
Nevertheless, patients receiving a sirolimus-based regimen had a significant decreased fathered pregnancy rate, compared with those administered sirolimus-free regimen (5.9 vs 92.9 pregnancies/1000 patient-years). Thus, men who desire to father children must be well educated of the risks and benefits associated with exposure to sirolimus. The underlying mechanisms still remains obscure. Feng et al. had found that SRL assumes a focal inhibitory part on a stem cell factor (SCF)/c-kit-dependent process in spermatogonial proliferation via the PI3- K/AKT/p70S6K pathway in the animal models [96-100].
In females, sirolimus may cause infertility, menstrual irregularities, secondary amenorrhea, and increases in luteinizing hormone and follicle-stimulating hormone. Boobes et al. were the first to demonstrate the suppressive effect of SRL on the ovaries of renal transplant recipients. Further study by Yu et al. had proved that inhibition of mTORi in granulosa cells and ovarian follicles resulted in compromised granulose proliferation and reduced follicle growth. Moreover, Yu and his colleagues detected increased fraction of cells that underwent anomalous mitotic events during mTOR inhibition in animal models [100].
The potentiality for everolimus to cause infertility in male and female patients is not known yet, however, male infertility and secondary amenorrhea have been observed [95].
Renal Nephrotoxicity
Although m-TOR-I were considered for long time as non-nephrotoxic drugs, however, experimental studies had shown that mTOR-inhibitors can influence renal structure and function, though their nephrotoxicity is much less than CNIs nephrotoxicity [1,4,29].
Following effects were noted with m-TOR-I:
1. mTOR-inhibitors can impairs recovery from ischemia in animal models of renal transplantation.
2. mTOR-inhibitors has synergistic effects if combined with CNI, and can worsen the CNI toxicity.
3. Delayed graft function is often seen with mTOR-inhibitors. Literatures have reported that patients on SRL stayed on dialysis for longer time compared to others and this was attributed to increase frequency of acute tubular necrosis, however, recent literature suggested that the hazard risk may be lower with EVR, due to the differences in pharmacokinetics between each and the lack for the loading doses in EVR.
4. New-onset proteinuria has been accounted for in two-thirds of patients converted to SRL. Histological lesions in biopsied patients resemble focal segmental glomerulosclerosis; in spite of the fact that it is not clear whether this is recurrent primary kidney disease, chronic allograft damage or happens as a result of CNI withdrawal and/or SRL introduction, therefore baseline proteinuria should be measured before any change regimen. It is conceivable that withdrawal of CNIs prompts hyperfiltration on the grounds that these medications have constrictive impacts on the renal vasculature. Reasons for proteinuria caused by SRL therapy could be explained by hemodynamic changes, podocyte injury, tubular dysfunction and antagonism of vascular endothelial growth factor.
5. Recent literatures had suggested that mTOR-I-induced proteinuria through reduction of glomerular nephrin expression in transplanted patients. It's advisable to add either ACEIs or ARBs when convert patients from CNIs to sirolimus for their vasoconstrictive properties on glomerular efferent arterioles [1,3,4,29,42].
6. Everolimus has similar effects on increasing proteinuria, and reversibility has been observed when patient discontinued the medicine and switched back to CNI therapy [26,27].
7. Renal graft thrombosis: An increased risk of kidney arterial and venous thrombosis, resulting in graft loss, has been reported, mostly within the first 30 days post-transplantation [26,27].
8. Tubular dysfunction: biochemical disorders like hypokalemia, hypophosphatemia, hypocalcaemia, and hypouricaemia was noted in some patients following switching from CNI to sirolimus, suggesting chronic tubular toxicity. Sirolimus therapy might alter tubular handling of K, phosphate and uric acid by renal tubules [1,3,15]. Renal magnesium wasting and potentially significant hypomagnesemia was also noted. Viorica Bumbea et al, interestingly had found that the renal phosphate threshold and uric acid clearance were altogether lower in patients taking sirolimus compared to those taking everolimus, despite similar PTH levels [3].
Special Considerations
Vaccinations
Immunosuppressants may affect the response to vaccination, rendering it to be less effective. Usage of live vaccines should be avoided [26].
Impact of mTORi on pregnancy
There are inadequate data regarding the use of m-TOR I in pregnant women. Studies in animals have shown reproductive toxicity including embryo/foetotoxicity. The potential risk for humans is unknown.
There are only 11 cases of transplanted women who received SRL during pregnancy were reported in literatures, hence the real safety has not yet been determined and there is no real existing data to strongly confirm that SRL/EVL are teratogenic. In one of those cases, SRL treatment was introduced at 24 weeks of pregnancy and the infant was born with cleft lip-palate and microtia. The first reported case of successful delivery in a renal-transplant female receiving SRL (in association with CNI) through all gestation was reported by Guardia et al. in 2006, subsequently in 2008 and 2011 two cases of successful delivery in a renal transplant females treated with SRL were reported by Chu et al. and Framarino-dei-Malatesta respectively. With regards to EVR there is, as SRL, little experience about its role during pregnancy. Only two cases of successful pregnancies in 2 kidney-transplant recipients treated with EVR during pregnancy were reported. All these cases demonstrate that either SRL or EVR might not be an absolute contraindication for pregnancy, however, due to limited available data most nephrologists prefer to avoid m-TOR I therapy completely during pregnancy and advise to be discontinued and switched to CNI at least 8-12 weeks prior to attempted conception unless the potential benefit outweighs the potential risk for the fetus [24,26-28,100].
Impact of mTORi on breast-feeding
Due to the potentiality for adverse reactions in the nursing infant, breast-feeding is not recommended by the manufacturer during therapy and for 2 weeks following the last dose [26,27].
Conclusion
mTOR-inhibitors are very promising immunosuppressive agents, It has different mode of action with different adverse effect profile and can be used as de novo therapy or as a substitute immunosuppressant mainly to CNI.
The promising effect of reduced nephrotoxicity compared to CNI, hypertension and lower incidence of post-transplant malignancy in addition to the lower viral rate infections; all had make them a good alternative to CNI therapy, however, delayed wound healing, lymphoceles and hematological disorders remained a great challenge with this therapy.
Furthermore, mTOR I has different implication outside transplant field, though their use remained off labeled in most. There is accumulating evidence that the mTOR pathway is involved in the pathophysiology of a number of neurological, genetic and inflammatory diseases that brand mTOR I as promising and novel agent in such diseases, however in most; this evidence is derived from basic science and animal models.
References
- Morath C, Arns W, Schwenger V, Mehrabi A, Fonouni H, et al. (2007) Sirolimus in renal transplantation. Nephrol Dial Transplant 22 Suppl 8: viii61-61viii65.
- Russ GR (2013) Optimising the use of mTOR inhibitors in renal transplantation. Transplant Res 2: S4.
- Bumbea V, Kamar N (2005) Long-term results in renal transplant patients with allograft dysfunction after switching from calcineurin inhibitors to sirolimus. Nephrol Dial Transplant 20: 2517–2523.
- Patel SJ, Dawson KL (2011) The Role of mTOR Inhibition in Renal Transplant Immune Suppression. Dialysis & Transplantation.
- Neuhaus P, Klupp J, Langrehr JM (2001) Review article, mTOR Inhibitors: An Overview. Liver Transplantation 7: 473-484.
- Bjorn Nashan (2004) Maximizing the clinical outcome with mTOR inhibitors in the renal transplant recipient: defining the role of calcineurin inhibitors. Transpl Int 17:179-285.
- Nguyen C, Shapiro R (2014) New immunosuppressive agents in pediatric transplantation. CLINICS 69: 8-16.
- Hardinger K, Brennan DC (2015) Mechanistic target of rapamycin (mTOR) inhibitors in renal transplantation.
- Groth CG, Backman L (1999) Sirolimus (Rapamycin)- based therapy in human renal transplantation. Similar Efficacy and different toxicity compared with Cyc. Transplantation. 67: 1036-1042.
- Samaniego M, Becker BN, Djamali A (2006) Drug Insight: maintenance immunosuppression in kidney transplant recipients. Nature Clinical Practice Nephrology 2: 688-699.
- Wullschleger S, Loewith R, Hall MN (2006) TOR signaling in growth and metabolism. Cell 124: 471-484.
- Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, et al. (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6: 1122-1128.
- Pópulo H, Lopes JM, Soares P (2012) The mTOR Signalling Pathway in Human Cancer. International Journal of Molecular Sciences. 13: 1886–1918.
- Meric-Bernstam F, Gonzalez-Angulo AM (2009) Targeting the mTOR signaling network for cancer therapy. J Clin Oncol 27: 2278-2287.
- Zaza G, Tomei P (2013) Systemic and Non renal Adverse Effects Occurring in Renal Transplant Patients Treated with mTOR Inhibitors. Review Article. Clinical and Developmental Immunology 2013:13.
- Garg S, Bourantas C, Serruys PW (2013) New concepts in the design of drug-eluting coronary stents. Nature Reviews Cardiology 10: 248-260.
- Hartmann B, Schmid G (2005) Biochemical monitoring of mTOR inhibitor–based immunosuppression following kidney transplantation: A novel approach for tailored immunosuppressive therapy. Kidney International 68 : 2593–2598.
- Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER). Rapamune (sirolimus) Oral Solution: Medical Officer’s Review and Clinical Pharmacology and Biopharmaceutics Review(s).
- MacDonald A, Scarola J, Burke JT, Zimmerman JJ (2000) Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther 22 Suppl B: B101-121.
- Mahalati K, Kahan BD (2001) Clinical pharmacokinetics of sirolimus. Clin Pharmacokinet 40: 573-585.
- Sirolimus (2016) Drug information. Up to date.
- Diekmann F, Campistol JM (2006) Conversion from calcineurin inhibitors to sirolimus in chronic allograft nephropathy: benefits and risks. Nephrol Dial Transplant 21: 562-568.
- Clinical Guidelines for Transplant Medications (2015).
- Hardinger K, Brennan DC (2016) Pharmacology of mammalian (mechanistic) target of rapamycin (mTOR) inhibitors.
- Vincenti F (2012) CNI Sparing With mTOR Inhibitors in Kidney Transplantation. Medscape Education Transplantation.
- Everolimus Novartis tablet ENG SmPC.
- Everolimus (2016) Drug information. Up to date.
- Kirchner GI, Meier-Wiedenbach I, Manns MP (2004) Clinical pharmacokinetics of everolimus. Clin Pharmacokinet 43: 83-95.
- Issa N, Braun WE (2008) Immunosuppression for Renal Transplant Patients and Common Medical Problems in Renal Transplantation. In: Carey WD (ed.) "Current Clinical Medicine 2009: Expert Consult Premium Edition". Cleveland Clinic Guides. 9: 897.
- Oberbauer R, Kreis H, Johnson RW (2003) Rapamune Maintenance Regimen Study Group. Long-term improvement in renal function with sirolimus after early cyclosporine withdrawal in renal transplant recipients: 2-year results of the Rapamune Maintenance Regimen Study. Transplantation 76: 364-370.
- Teperman L, Moonka D, Sebastian A, Sher L, Marotta P, et al. (2013) Spare-the-Nephron Trial Liver Transplantation Study Group. Calcineurin inhibitor-free mycophenolate mofetil/sirolimus maintenance in liver transplantation: the randomized spare-the-nephron trial. Liver Transpl 19: 675-689.
- Lebranchu Y, Thierry A, Toupance O, Westeel PF, Etienne I et al. (2009) Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transplant 9: 1115-1123.
- Schena FP, Pascoe MD (2009) Conversion From Calcineurin Inhibitors to Sirolimus Maintenance Therapy in Renal Allograft Recipients: 24-Month Efficacy and Safety Results From the CONVERT Trial. Transplantation 87: 2.
- Budde K, Becker T, Arns W, Sommerer C, Reinke P, et al. (2011) ZEUS Study Investigators: Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomised, controlled trial. Lancet 377: 837-847.
- Holdaas H, Rostaing L, Serón D, Cole E, Chapman J, et al. (2011) ASCERTAIN Investigators Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 92: 410-418.
- Mjornstedt L, Sorensen SS, von Zur Muhlen B (2014) Renal function three years after early conversion from a calcineurin inhibitor to everolimus: Results from a randomized trial in kidney transplantation. Transplant International 28: 42-51.
- Egidi MF, Cowan PA, Naseer A, Gaber AO (2003) Conversion to sirolimus in solid organ transplantation: a single-center experience. Transplant Proc 35: 131S-137S.
- Diekmann F, Budde K, Oppenheimer F, Fritsche L, Neumayer HH, et al. (2004) Predictors of success in conversion from calcineurin inhibitor to sirolimus in chronic allograft dysfunction. Am J Transplant 4: 1869-1875.
- Oberbauer R, Segolini G, Campistol JM (2005) Early cyclosporine withdrawal from a sirolimus-based regimen results in better renal allograft survival and renal function at 48 months after transplantation. Transplant Int 18: 22-28.
- Renders L, Steinbach R, Valerius T , Schocklmann HO, Kunzendorf U (2004) Low dose sirolimus in combination with mycophenolate mofetil improves kidney graft function late after renal transplantation and suggests pharmacokinetic interaction of both immunosppresive drugs. Kidney Blood Press Res 27: 181-185.
- Special Issue (2009) KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Chapter 5: Monitoring Immunosuppressive Medications. American Journal of Transplantation 9: S19–S20.
- Johnson RWG, Kreis H (2001) Sirolimus Allows Early Cyclosporine Withdrawal In Renal Transplantation Resulting In Improved Renal Function And Lower Blood Pressure. Transplantation 72: 777–786.
- Piselli P, Busnach G, Citterio F, Richiardi L, Cimaglia C (2008) Kidney transplant and cancer risk: an epidemiological study in Northern and Central Italy. [Article in Italian]- English abstract. Epidemiol Prev 32: 205-211.
- Campistol JM, Gutierrez-Dalmau A, Torregrosa JV (2004) Conversion to sirolimus: a successful treatment for posttransplantation Kaposi's sarcoma. Transplantation 77: 760-762.
- Luan FL, Hojo M, Maluccio M, Yamaji K, Suthanthiran M (2002) Rapamycin blocks tumor progression: unlinking immunosuppression from antitumor efficacy. Transplantation 73:1565-1572.
- Huang S, Houghton PJ (2003) Targeting mTOR signaling for cancer therapy. Current Opinion in Pharmacology 3: 371–377.
- Wander SA, Hennessy BT, Slingerland JM (2011) Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest 121: 1231–1241.
- Brachmann S, Fritsch C, Maira SM (2009) PI3K and mTOR inhibitors—a new generation of targeted anticancer agents. Current Opinion in Cell Biology 21: 194-198.
- Zhang YJ, Duan Y, Zheng XF (2011) Targeting the mTOR kinase domain: the second generation of mTOR inhibitors. Drug Discov Today 16: 325-331.
- Wander SA, Hennessy BT, Slingerland JM (2011) Next-generation mTOR inhibitors in clinical oncology: How pathway complexity informs therapeutic strategy. Journal of Clinical Investigation 121: 1231–1241.
- Tanneeru K, Guruprasad L (2011) Ligand-based 3-D pharmacophore generation and molecular docking of mTOR kinase inhibitors. Journal of Molecular Modeling18: 1611-1624.
- Ballou LM, Lin RZ (2008) Rapamycin and mTOR kinase inhibitors. J Chem Biol 1: 27-36.
- Sutherlin DP, Bao L, Berry M, Castanedo G, Chuckowree I, et al. (2011) Discovery of a Potent, Selective, and Orally Available Class I Phosphatidylinositol 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Kinase Inhibitor (GDC-0980) for the Treatment of Cancer. Journal of Medicinal Chemistry 54: 7579-7587.
- Zhou H, Luo Y, Huang S (2010) Updates of mTOR inhibitors. Anticancer Agents Med Chem 10: 571-581.
- Song HG, Park DW, Kim YH, Ahn JM, Kim WJ, et al. (2012) Randomized trial of optimal treatment strategies for in-stent restenosis after drug-eluting stent implantation. J Am Coll Cardiol 59:1093-1100.
- Park DW, Kim YH, Song HG, Ahn JM, Kim WJ, et al. (2011) Comparison of everolimus- and sirolimus-eluting stents in patients with long coronary artery lesions: a randomized LONG-DES-III (Percutaneous Treatment of LONG Native Coronary Lesions With Drug-Eluting Stent-III) Trial. JACC Cardiovasc Interv 4:1096-1103.
- Pahon E (2015) FDA approves Rapamune to treat LAM, a very rare lung disease. FDA.gov. U.S. Food and Drug Administration.
- Lister Hill National Center for Biomedical Communications U.S. National Library of Medicine National Institutes of Health Department of Health & Human Services. Genetics Home Reference.
- Ferrans VJ, Yu ZX, Nelson WK, Valencia JC, Tatsuguchi A, et al. (2000) Lymphangioleiomyomatosis (LAM): a review of clinical and morphological features. J Nippon Med Sch 67: 311-329.
- Caron A, Richard D, Laplante M (2015) The Roles of mTOR Complexes in Lipid Metabolism. Annu Rev Nutr 35: 321-348.
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, et al. (2006) Hypothalamic mTOR signaling regulates food intake. Science 312: 927-930.
- Kriete A, Bosl WJ, Booker G (2010) Rule-based cell systems model of aging using feedback loop motifs mediated by stress responses. PLoS Comput Biol 6: e1000820.
- Magnuson B, Ekim B, Fingar DC (2012) Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. The Biochemical Journal 441: 1-21.
- Jeong A, Wong M (2016) mTOR Inhibitors in Children: Current Indications and Future Directions in Neurology. Curr Neurol Neurosci Rep 16: 102.
- Huang X, Zhang H, Yang J, Wu J, McMahon J, et al. (2010) Pharmacological inhibition of the mammalian target of rapamycin pathway suppresses acquired epilepsy. Neurobiol Dis 40: 193-199.
- Van Vliet EA, Forte G, Holtman L, den Burger JC, Sinjewel A, et al. (2012) Inhibition of mammalian target of rapamycin reduces epileptogenesis and blood brain barrier leakage but not microglia activation. Epilepsia 53:1254-1263.
- Buckmaster PS, Lew FH (2011) Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci 31: 2337-2347.
- Sliwa A, Plucinska G, Bednarczyk J, Lukasiuk K (2012) Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci Lett 509: 105-109.
- Heimberger AB, Wang E, McGary EC, Hess KR, Henry VK, et al. (2005) Mechanisms of action of rapamycin in gliomas. Neuro Oncol 7: 1-11.
- Nanoparticles treat muscular dystrophy in mice | Newsroom | Washington University in St. Louis. News.wustl.edu.
- Bibee KP, Cheng YJ, Ching JK (2014) Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. The FASEB Journal 28: 2047-2061.
- Blagosklonny MV (2011) Progeria, rapamycin and normal aging: recent breakthrough. Aging (Albany NY) 3: 685-691.
- Fernandez D, Bonilla E, Mirza N, Niland B, Perl A (2006) Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis & Rheumatism 54: 2983-2988.
- Fernandez D, Perl A (2010) mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus? Discov Med 9: 173-178.
- Lai ZW, Borsuk R, Shadakshari A, Yu J, Dawood M, et al. (2013) Mechanistic target of rapamycin activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol 191: 2236-2246.
- Mitra A, Luna JI, Marusina AI, Merleev A, Kundu-Raychaudhuri S, et al. (2015) Dual mTOR Inhibition Is Required to Prevent TGF-β-Mediated Fibrosis: Implications for Scleroderma. J Invest Dermatol 135: 2873-2876.
- Yi H, Brooks ED, Thurberg BL, Fyfe JC, Kishnani PS, et al. (2014) Correction of glycogen storage disease type III with rapamycin in a canine model. J Mol Med (Berl) 92: 641-650.
- Rosner M, Hanneder M, Siegel N, Valli A, Fuchs C, et al. (2008) The mTOR pathway and its role in human genetic diseases. Mutat Res 659: 284-292.
- Braun WE, Schold JD, Stephany BR, Spirko RA, Herts BR (2014) Low-dose rapamycin (sirolimus) effects in autosomal dominant polycystic kidney disease: an open-label randomized controlled pilot study. Clin J Am Soc Nephrol 9: 881-888.
- He Q, Lin C, Ji S, Chen J (2012) Efficacy and safety of mTOR inhibitor therapy in patients with early-stage autosomal dominant polycystic kidney disease: a meta-analysis of randomized controlled trials. Am J Med Sci 344: 491-497.
- Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, et al. (2006) TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126: 955-968.
- Chan AY, Soltys CL, Young ME, Proud CG, Dyck JR (2004) Activation of AMP-activated protein kinase inhibits protein synthesis associated with hypertrophy in the cardiac myocyte. J. Biol. Chem 279: 32771–32779.
- Dann SG, Selvaraj A, Thomas G (2007) mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med 13: 252-259.
- Inoki K, Corradetti MN, Guan KL (2005) Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet 37: 19-24.
- Donia M, McCubrey JA, Bendtzen K, Nicoletti F (2010) Potential use of rapamycin in HIV infection. Br J Clin Pharmacol 70: 784-793.
- Powers RW 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S (2006) Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev 20: 174-184.
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, et al. (2011) Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 66: 191-201.
- Tardif S, Ross C, Bergman P, Fernandez E, Javors M, et al. (2015) Testing efficacy of administration of the antiaging drug rapamycin in a nonhuman primate, the common marmoset. J Gerontol A Biol Sci Med Sci 70: 577-587.
- Check Hayden E (2014) Pet dogs set to test anti-ageing drug. Nature 514: 546.
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, et al. (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392-395.
- Lamming DW (2014) Diminished mTOR signaling: a common mode of action for endocrine longevity factors. Springerplus 3: 735.
- Schieke SM, Phillips D, McCoy JP, Aponte AM, Shen RF, et al. (2006) The Mammalian Target of Rapamycin (mTOR) Pathway Regulates Mitochondrial Oxygen Consumption and Oxidative Capacity. J Biol Chem 281: 27643-27652.
- Aboujaoude W, Milgrom ML, Govani MV (2004) Lymphedema associated with sirolimus in renal transplant recipients. Transplantation 77: 1094-1096.
- Lee S, Coco M, Greenstein SM, Schechner RS, Tellis VA, et al. (2005) The effect of sirolimus on sex hormone levels of male renal transplant recipients. Clin Transplant 19: 162-167.
- Kaczmarek I, Groetzner J, Adamidis I, Landwehr P, Mueller M, et al. (2004) Sirolimus impairs gonadal function in heart transplant recipients. Am J Transplant 4: 1084-1088.
- Tondolo V, Citterio F, Panocchia N, Nanni G, Favi E, et al. (2005) Gonadal function and immunosuppressive therapy after renal transplantation. Transplant Proc 37: 1915-1917.
- Huyghe E, Zairi A, Nohra J, Kamar N, Plante P, et al. (2007) Gonadal impact of target of rapamycin inhibitors (sirolimus and everolimus) in male patients: an overview. Transpl Int 20: 305-311.
- Framarino-dei-Malatesta M, Derme M, Manzia TM, Iaria G, De Luca L, et al. (2013) Impact of mTOR-I on fertility and pregnancy: state of the art and review of the literature. Expert Rev Clin Immunol 9: 781-789.
- Boobes Y, Bernieh B, Saadi H, Raafat Al Hakim M, Abouchacra S (2010) Gonadal dysfunction and infertility in kidney transplant patients receiving sirolimus. Int Urol Nephrol 42: 493-498.
- Yu J, Yaba A, Kasiman C, Thomson T, Johnson J (2011) mTOR controls ovarian follicle growth by regulating granulosa cell proliferation. PLoS One 6: e21415.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences