Less Known Early Complication of Baricitinib Use: Acute Kidney Injury
Lakshmi Kannan
Lakshmi Kannan*
Department of Nephrology, Pikeville Medical Center, Kentucky, USA
- *Corresponding Author:
- Lakshmi Kannan
Department of Nephrology,
Pikeville Medical Center,
Kentucky,
USA,
E-mail: lakshmi.kannan@pikevillehospital.org
Received Date: December 24, 2021; Accepted Date: January 7, 2022; Published Date: January 14, 2022
Citation: Kannan L (2022) Less Known Early Complication of Baricitinib Use: Acute Kidney Injury. J Clin Exp Nephrol Vol.6 No:6: 119.
Abstract
Historically Baricitinib has been used to treat patients with Rheumatoid Arthritis. Recently, FDA has approved its use in the treatment of COVID-19 in hospitalized patients (2 years and older) requiring supplemental oxygen, mechanical ventilation or extracorporeal membrane oxygenation as part of Emergency Use Authorization as more studies show that the substantial morbidity and mortality due to COVID19 may be due to a dysregulated inflammatory response. Though it is more frequently used now either alone or with remdesivir, not much is known about the complications/implications of Baricitinib and parameters that need to be monitored.
Keywords
Acute kidney injury; Baricitinib; Creatinine; Jak- STAT; Rheumatoid arthritis
Introduction
Patients with severe COVID-19 infection often develop a hyperinflammatory state that lead to multi-organ dysfunction like Acute Respiratory Distress Syndrome (ARDS), secondary bacterial infection/sepsis, shock, acute kidney injury and even death [1]. Though traditionally, IV dexamethasone, Vitamin C and remdesivir have been used, significant mortality reduction is still unmet [2]. Baricitinib is a selective Janus kinase (JAK)1/JAK 2 inhibitor, known for its anti-inflammatory profile in patients with autoimmune disease [3]. Given its anticytokine properties, Baricitinib was identified as a potential intervention for the treatment of COVID-19[4] our case aims to emphasize the importance of monitoring serum creatinine level as a potential early complication of Baricitinib use.
Case Presentation
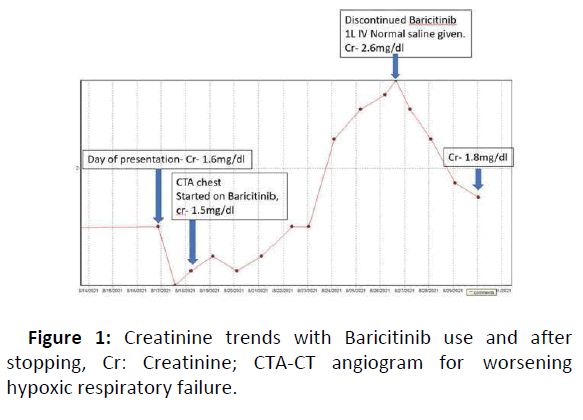
A 71-year-old white female with medical history significant for hypertension, Type II Diabetes Mellitus and chronic kidney disease Stage III (Baseline creatinine 1.4-1.6 mg/dl) presented to the hospital for worsening shortness of breath, fever and cough for 2 weeks. In the Emergency room, the patient was hypoxic with oxygen saturation of 90% on room air and 92% on 4 L nasal cannula oxygen. Chest X-ray showed basilar and perihilar infiltrates concerning for pneumonia and she tested positive for SARS-CoV-2. She was started on IV dexamethasone 6 mg daily, Vitamin C 500 mg daily, Doxycycline and ceftriaxone. Over the next 24 hours, the patient’s respiratory status worsened with increasing oxygen requirements up to 12 L. She had a CTA chest on hospital Day 2, which showed extensive multifocal bilateral infiltrates and mild pulmonary vascular congestion. Infectious Disease specialist (ID) was consulted as her initial labs showed significantly elevated acute phase reactants (ESR>130, CRP 16.5, ferritin 1387 ng/ml, and leukocytosis) concerning for hyper inflammatory syndrome. Initially, the patient refused remdesivir because of its side effect profile in chronic kidney disease so she was started on Baricitinib 4 mg daily per ID recommendations. On Day 4, her creatinine went up slightly to 1.6 mg/dl after an initial dip to 1.4 mg/dl the day before as shown in Figure 1, likely consistent with contrast use (received 53 ml of ISOVUE 370 Inj).
The patient was also started on IV bumex 1 mg twice daily for vascular congestion. On the 6th day, the patient’s creatinine started to trend up from 2.2 mg/dl to 2.6 mg/dl at which point Baricitinib was held along with bumex and 1 L IV normal saline was given. She received a total of 9 days of Baricitinib (total dose-36 mg). Creatinine started to improve 24 hours later and continued to improve back to baseline of 1.6 mg/dl within 3 days.
The patient continued to improve from respiratory standpoint just with steroids and she was discharged home.
Results and Discussion
Baricitinib is a reversible Janus- associated kinase (JAK) inhibitor with particular affinity to JAK1 and JAK2. SARS-CoV-2 infection can induce the release of interleukin (IL)-1, IL-6, tumor necrosis factor and other cytokines during the critical phase of COVID-19 infection resulting in “cytokine storm”. The JAK/Signal Transducers and Activators of Transcription (STAT) pathway mediate cytokine signaling pathway by potentially attenuating cytokine production or block cytokine-receptor activation and inhibit lysosomal activity [5].
The kidney excretes approximately 75% of the drug and risk of adverse events increases with reduced/declining renal function. Known complications of Baricitinib (from its use for rheumatoid arthritis) include anemia, life threatening infections, malignancies like lymphoma and thrombosis. Its study of adverse effects in the treatment of COVID-19 has not been established yet [6]. There are limited data on the use of Baricitinib in patients with severe renal impairment including those on dialysis or AKI with creatinine clearance less than 30 ml/min.
In the case presented above, no factor could be linked to/ implicated in the rise in creatinine other than Baricitinib use. After an initial trend up and down of creatinine in a span of 48 hours of using IV contrast for CTA, the creatinine continued to steadily rise with continued Baricitinib use. Also, the fact that creatinine went down immediately (within 24 hours) after discontinuing Baricitinib made it a strong reason to suspect Baricitinib induced creatinine elevation.
Baricitinib induced creatinine elevation could be potentially explained from the JAK/STAT signaling pathways: JAK/STAT expression, specifically the isoforms- JAK2 and STAT3 and activation occur in varied kidney diseases [7]. Their role appears to be spatially and temporally dependent. For example, JAK/STAT signaling contribute to the pathogenesis of diabetic kidney disease (DKD), ADPCKD and HIVAN but appears to play a protective role in AKI, especially activation of STAT3 in renal proximal tubular epithelial cells may have a protective role after ischemia-reperfusion injury. Knock-out mice models have shown that overexpression of STAT3 in cultured tubular epithelial cells improve cell survival and reduce apoptosis [8]. Baricitinib via its mechanism of action inhibits JAK/STAT signaling pathway which takes away the protective role of STAT3 in kidneys which may lead to acute kidney injury.
The other possible mechanism of Baricitinib induced creatinine elevation is through its ability to inhibit renal tubular secretion of creatinine [9].
Conclusion
Baricitinib having a short half-life and its ability to act on targeted inflammatory pathways while minimizing biologic redundancy with less immunosuppression has shown promising results in reducing recovery time and accelerating improvement in clinical status especially in patients with hyper inflammatory syndrome. This case highlights the importance of monitoring for short-term and long-term side effects with Baricitinib use and emphasizes the protective role of STAT3 in AKI but further studies involving the role of STAT3 activation or inhibition through Baricitinib in acute kidney injury remains to be investigated.
Disclosure
Written informed consent was obtained from the patient for publication of this case report.
References
- Siddiqi HK (2020) COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 39: 405-407
- Beigel JH (2020) Remdesivir for the treatment of COVID-19-final report. N Engl J Med 383: 1813-1826
- Sanchez GAM (2018) JAK1/2 inhibition with baricitinib in the treatment of autoinflammatory interferonopathies. J Clin Invest 128: 3041-3052
- Richardson P (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet 395: e30-e31
- Baricitinib: FDA Emergency Use Authorization and Potential Role in Management of COVID 19. Pennsylvania Society of Health Systems Pharmacists. 2021
- Jorgensen S, Tse C, Burry L, Dresser L (2020) Baricitinib: A review of pharmacology, safety and emerging clinical experience in COVID‐19. Pharmacotherapy 40: 843-856.
- Chuang P, He J (2010) JAK/STAT signaling in renal diseases. Kidney Int 78: 231-234.
- Pace J, Paladugu P, Das B, He J, Mallipattu S (2019) Targeting STAT3 signaling in kidney disease. 316: F1151-F1161.
- Summary of product characteristics. European Commission. 2018
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences