HLA-class Ib expression suppresses neutrophil xenogeneic immune responses against pig cells
DOI10.36648/2472-5056.10.1.292
Koki Takase1, Katarzyna Gadomska1, Akira Maeda1, Jun Matsui1, Kengo Nakahata1, Motonari Nomura1, Masafumi Kamiyama1, Takehisa Ueno1, Shunsuke Saito2, Akihiro Ike3, Soichi Matsumura4, Yoichi Kakuta4, Hiroshi Eguchi1, Hiroomi Okuyama1 and Shuji Miyagawa1*
1Department of Pediatric Surgery, Osaka University Graduate School of Medicine, Suita, Japan
2Department of Cardiovascular Surgery, Osaka University Graduate School of Medicine, Suita, Japan
3Department of General Thoracic Surgery, Osaka University Graduate School of Medicine, Suita, Japan
4Department of Urology, Osaka University Graduate School of Medicine, Suita, Japan
- *Corresponding Author:
- Shuji Miyagawa
Department of Pediatric Surgery, Osaka University Graduate School of Medicine, Suita, Japan
E-mail: miyagawa@pedsurg.med.osaka-u.ac.jp
Received date: December 31, 2024, Manuscript No. IPJCEN-25-20271; Editor assigned date: January03, 2025, PreQC No. IPJCEN-25-20271(PQ); Reviewed date: January17, 2025, QC No. IPJCEN-25-20271; Revised date: January24, 2025, Manuscript No. IPJCEN-25-20271(R); Published date: January31, 2025, DOI: 10.36648/2472-5056.10.1.292
Citation: Takase K, Gadomska K, Maeda A, Matsui J, Nakahata K, et al. (2025) HLA-Class Ib Expression Suppresses Neutrophil Xenogeneic Immune Responses Against Pig Cells. J Clin Exp Nephrol Vol.10 No.1: 292.
Abstract
Purpose: The expression of Human Leukocyte Antigen (HLA) class â? molecules regulates innate immune responses of Natural Killer (NK) cells and macrophages. In this study, we investigated the regulatory responses of Human Leukocyte Antigen G1(HLA-G1) and Human Leukocyte Antigen E (HLAE) on human neutrophils.
Material and methods: The expression of counter receptors on neutrophils was detected using flow cytometry. Complementary DNA (cDNA) of HLA-G1 and HLA-E were introduced into Swine Endothelial Cells (SECs) to establish SEC/HLA-G1 and SEC/HLA-E cell lines, respectively. These cell lines were then co-cultured with neutrophils to assess cytotoxicity. Subsequently, Reactive Oxygen Species (ROS) production levels were calculated using Cell Reactive Oxygen Green (CellROX Green). Neutrophil Extracellular Traps (NETosis) in neutrophils of SEC cells was measured using SYTOX Green.
Results: The expression of Natural Killer Group 2 member A(NKG2A), Immunoglobulin-Like Transcript 2 (ILT-2) and ILT-4 on neutrophils was <20%, approximately 30% and approximately 65%, respectively. No significant differences in cytotoxicity were observed in SEC/HLA-E and wild-type SEC cells, whereas SEC/HLA-G1 cells exhibited significant reduction of approximately 35% and 25% in the cytotoxicity after 4 h of incubation with Phorbol 12-Myristate 13- Acetate (PMA) and 24 h of incubation without PMA, respectively. Additionally, SEC/HLA-G1 cells significantly inhibited ROS production. HLA-G1 also suppresses NETosis induction in neutrophils.
Conclusion: These results indicate that HLA-G1 effectively regulates the xenogeneic immune responses of neutrophils against SECs in xenotransplantation.
Keywords
HLA-G1; Xenotransplantation; Neutrophil; Innate immunology; Internal ribosome entry site; Human beta-2 microglobulin (hβ2m)
Introduction
Several Genetically Edited (GE) pigs have been developed and used for xenotransplantation [1]. The six transgenes in “10GEpigs” contain two complement regulatory proteins, two anticoagulant factors, one anti-inflammatory molecule and Closs of Differentiation 47 (CD47), a factor that regulates monocytes and macrophages [2,3]. Another venture company developed pig lines expressing CD47 and Human Leukocyte Antigen E (HLA-E), but their expression levels were insufficient [4].
After years of basic research and preclinical experiments using non-human primates and brain-dead recipients, it has become evident that hyperacute rejection can be overcome using GEpigs along with medication [5-10].
Despite this, gene editing in these pigs is insufficient and acute rejection follows. It is important to regulate innate immune cells, including Natural Killer (NK) cells, monocytes/ macrophages and neutrophils, to suppress secondary rejection reactions [11-14].
Regarding the regulation of these innate immune cells in xenotransplantation research, the issue of graft damage caused by NK cells has become a key focus area from an early stage. The introduction of HLA class Ib into pig grafts has received attention as a potential solution to this issue.
The next challenge was to regulate monocytes and macrophages. In this regard, the expression of CD47 in grafts has been implemented and GE-pigs expressing CD47 have been developed. Issues associated with the expression of CD47 in GE pigs have been reported. A novel molecule, a hybrid of Collectin- P1 (CL-P1) and Surfactant Protein-D (SP-D) (CL/SP-D), can regulate the activity of macrophages better than CD47 in GE-pig cells [15,16]. Additionally, we previously reported that regulation of cells by recognizing HLA class Ib molecules such as HLA-G1 and HLA-E can be implemented for NK cells, monocytes/ macrophages [17,18].
In this study, we focused on neutrophils and examined whether HLA class Ib molecules exhibit regulatory functions on neutrophils. The expression of HLA-G1 and HLA-E counter receptors was assessed in human neutrophils. In addition, xenogeneic rejection of Swine Endothelial Cells (SECs) was investigated in an in vitro system.
Materials and Methods
Cells
MYP30 lineage SEC were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% heatinactivated Fetal Bovine Serum (FBS). Human peripheral blood neutrophils were isolated and kept in a humidified incubator at 37°C, 5% CO2, in Roswell Park Memorial Institute Medium (RPMI)medium containing 10% FBS [19].
Construction of the plasmids
cDNA was prepared for HLA-G1 alone and subcloned into the pCXN2L (β-actin promoter) expression vector. cDNAs for the HLA-Ev (147) (Human Leukocyte Antigen E Variant) and human β2-microglobulin(hβ2m) were also prepared [20]. Subsequently, the hybrid gene, HLA-Ev (147) +IRES+hβ2m, was constructed and cloned into the pCXN2L expression vector. All sequences were verified by means of an ABI 310 autosequencer (Perkin-Elmer Corporation, Norwalk, CT).
Establishment of the transfected cells
Plasmids encoding HLA-G1 and HLA-E were transfected into SECs by electroporation using the neon® transfection system (Thermo-Fisher Scientific, Tokyo, Japan). The transfected cells were then subjected to drug selection by adding G418 (NacalaiTesque, Kyoto, Japan) to the medium and clonal individuals of SEC/HLA-G1 and SEC/HLA-E were established by limiting dilution.
The SEC cells (1 x 106) were stained with a mouse monoclonal Antibody (mAb) against HLA-class I, B9.12.1 (CosmoBio, Tokyo, Japan) for 30 min at 4°C, followed by a secondary antibody, Fluorescein Isothiocyanate (FITC) conjugated rabbit anti-mouse IgG (Cappel ICN) for 30 min at 4°C.
The expression of counter inhibitory receptors on neutrophil was evaluated using flow cytometry. The expression levels of Immunoglobulin-Like Transcriptï¼?ILT-2, ILT-4) and NKG2A were assessed using Alexa Fluor 647 anti-human CD85j mAb (GHI/75) (Biolegend, San Diego, CA), Phycoerythrin (PE)-labeled antihuman CD85d mAb (42D1) (Biolegend) and PE-labeled antihuman NKG2A mAb (R and D Systems, Minesota, MN), respectively. The stained cells were analyzed using a FACS (Fluorescence-Activated Cell Sorting) Calibur flow cytometer (BD Biosciences, San Diego, CA).
Neutrophil isolation
Human peripheral blood mononuclear cells were isolated from healthy volunteer donors, followed by differential density gradient separation with Polymorphprep® (Alere Technologies AS, Oslo, Norway). The remaining erythrocytes were dissolved with a lysis buffer (0.83% NH4Cl, 10 mmol/L Hepes-NaOH, pH 7.4) and incubated at 37°C for 3 min. The isolated cells were labeled using PE-labeled anti-CD66b mAb (Bio Legend) and a purity of approximately 95% was confirmed by flow cytometry [21].
Cytotoxicity assay
Naive SEC, SEC/HLA-G1 and SEC/HLA-E were plated on day 0 at 37°C in flat-bottom gelatin-coated 96-well plates at a concentration of 1.0 x 104 cells/well. Neutrophils (1.0 x 105 cells/ well) were added to each well with 50 nmol/L Phorbol 12- Myristate 13-Acetate (PMA) on day 1 of experiment 1. Then, 10 μl of the Water-Soluble Tetrazolium-8 (WST-8) solution (NacalaiTesque) was added to each well and the absorbance was measured using a microplate reader (Thermo-Fischer Scientific). The %cytotoxicity was calculated as follows:
%cytotoxicity= (OD (co-culture)-OD (neutrophils or dHL-60))/ (OD(SEC)-OD (medium)) x 100 (%) [21].
In experiment 2, the same WST-8 assay was performed after 24 h of coculture without PMA.
Detection of Reactive Oxygen Species (ROS) in neutrophil
ROS levels were quantified after staining with CellROX™ Green Reagent (Thermo-Fischer Scientific). CellROX™ reagent was added to cells at a final concentration of 5 μM, followed by incubation in the dark at 37Ë?C for 30 min. Subsequently, the stained cells were resuspended in Phosphate-Buffered Saline (PBS) and fluorescence images were evaluated using a FACSVerse™ flow cytometer (BD Biosciences) [16].
NETosis assay by SYTOX green
The SECs were plated in 24 well dishes at a concentration of 4 × 104 cells/well. After 24 h of incubation, 4 × 105 neutrophils were mixed into individual wells; after 2.5 h of incubation in the presence of 50 nmol/L PMA, the cells were collected by gentle pipetting. The wells were stained with 30 nmol/L SYTOX Green (Thermo-Fischer Scientific) and PE-labeled anti-human CD66 antibodies for 15 min at room temperature. The percent value of NETosis was calculated as (the number of CD66b+SYTOX Green+ cells/the number of CD66b+ neutrophils) x 100 (%), using a FACSVerse™flow cytometer [16].
Statistically analysis
The results of each assay are presented as means and standard errors. Statistical significance was determined using a 2-tailed t test. Statistical significance was set at p<0.05.
Results
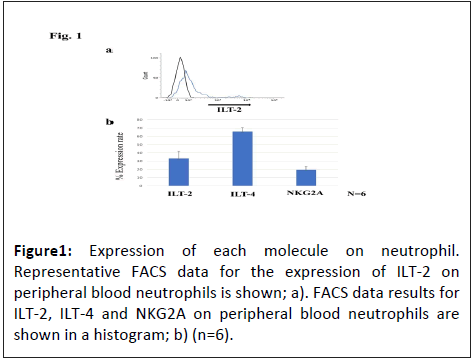
Expression levels of Immunoglobulin-Like Transcript 2 (ILT-2), ILT-4 and NKG2A on neutrophils
Human peripheral neutrophils were isolated and the expression levels of NKG2A/CD94, ILT-2 and ILT-4 were analysed by flow cytometry. The expression levels of NKG2A, ILT-2 and ILT-4 on neutrophils was 19.6% ± 9.9%, 33.2% ± 21.8% and 65.9% ± 11.6%, respectively (Figure 1).
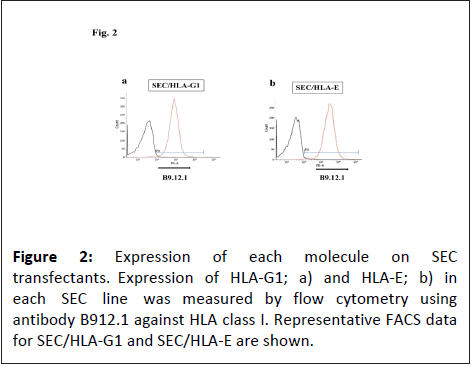
Expression levels of HLA-G1 and HLA-E in the transfected SECs
The expression levels of HLA class Ib in SECs were verified using flow cytometry. Developed SEC/HLA-G1 and SEC/HLA-E clones exhibited expression levels of 97% and 99% of HLA-G1 and HLA-E, respectively (Figure 2).
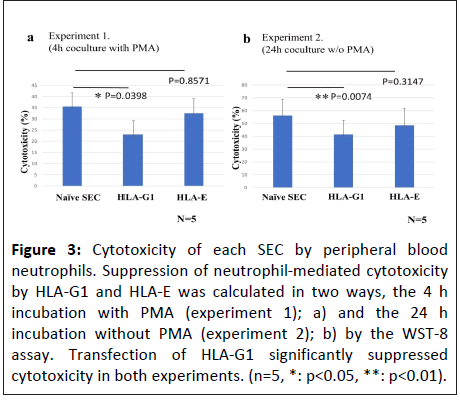
Cytotoxicity assay using peripheral blood neutrophils
Peripheral blood neutrophils were used as effector cells to assess their cytotoxicity against SECs. Transfection with HLA-G1 significantly suppressed cytotoxicity in both experiments, whereas the suppression of cytotoxic activity by HLA-E was not significant, corresponding to the expression rate of NKG2A (Figure 3).
Figure 3: Cytotoxicity of each SEC by peripheral blood neutrophils. Suppression of neutrophil-mediated cytotoxicity by HLA-G1 and HLA-E was calculated in two ways, the 4 h incubation with PMA (experiment 1); a) and the 24 h incubation without PMA (experiment 2); b) by the WST-8 assay. Transfection of HLA-G1 significantly suppressed cytotoxicity in both experiments. (n=5, *: p<0.05, **: p<0.01).
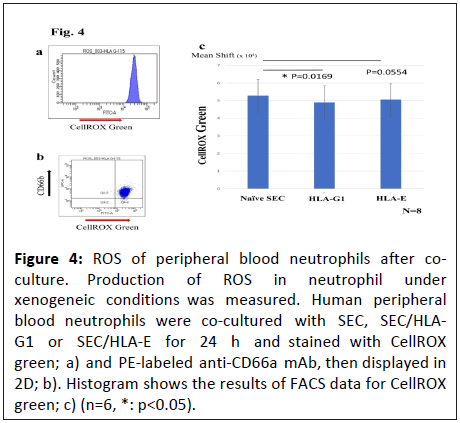
ROS production in peripheral blood neutrophils
ROS production was measured to examine the inhibitory effects of HLA-G1 and HLA-E on the innate immune responses of neutrophils. Although SEC/HLA-E cells showed a declining trend of ROS production in co-cultured neutrophils, a significant reduction in ROS production was induced by SEC/HLA-G1, indicating that HLA-G1 can inhibit ROS production in neutrophils (Figure 4).
Figure 4: ROS of peripheral blood neutrophils after coculture. Production of ROS in neutrophil under xenogeneic conditions was measured. Human peripheral blood neutrophils were co-cultured with SEC, SEC/HLAG1 or SEC/HLA-E for 24 h and stained with CellROX green; a) and PE-labeled anti-CD66a mAb, then displayed in 2D; b). Histogram shows the results of FACS data for CellROX green; c) (n=6, *: p<0.05).
NETosis of peripheral blood neutrophils
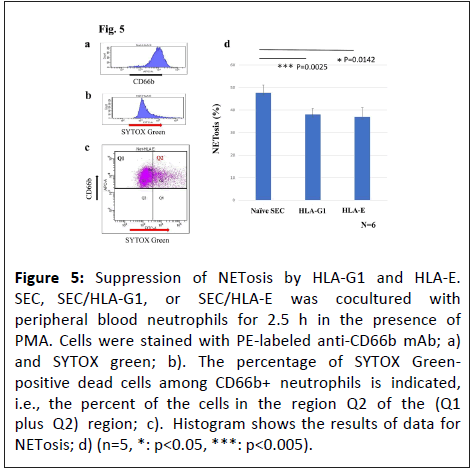
Peripheral blood neutrophils were stained with SYTOX Green after incubation of 2.5 h with SEC, SEC/HLA-G1, or SEC/HLA-E to assess the influence of HLA-G1 and HLA-E on NETosis. Both HLAG1 and HLA-E significantly inhibited NETosis (Figure 5).
Figure 5: Suppression of NETosis by HLA-G1 and HLA-E. SEC, SEC/HLA-G1, or SEC/HLA-E was cocultured with peripheral blood neutrophils for 2.5 h in the presence of PMA. Cells were stained with PE-labeled anti-CD66b mAb; a) and SYTOX green; b). The percentage of SYTOX Greenpositive dead cells among CD66b+ neutrophils is indicated, i.e., the percent of the cells in the region Q2 of the (Q1 plus Q2) region; c). Histogram shows the results of data for NETosis; d) (n=5, *: p<0.05, ***: p<0.005).
Discussion
Neutrophils are characterized by their fast response and extreme phagocytic ability compared with macrophages. In addition, neutrophils release various granules to attack the host, but it is very short-lived and usually replaced every 3-7 days.
Primarily, Fc and complement receptors trigger these reactions in neutrophils. However, the role of neutrophils in xenograft rejection has not been well studied. We previously reported for the first time that neutrophils can recognize and damage SECs, with CD31 and CD177 playing a regulatory role in this response. In addition, we investigated the regulating mechanism of neutrophils [21,22].
Xenotransplantation research began with a discovery of the species specificity of complement and Complement Regulatory Proteins (CRPs) [10]. Next, the focus shifted towards regulating xenogeneic carbohydrate antigens such as α-Gal, which were also implicated in the regulation of NK cells [23].
In the 1980s, the mechanism by which NK cells recognize themselves via HLA molecules was revealed, known as the "missing-self theory" [24]. Subsequently, inhibitory receptors that recognize HLA class I antigens and inhibit their harmful effects were discovered. NK receptors, including Killer Cell Immunoglobulin-Like Receptors (KIRs), Immunoglobulin-Like Receptors (LILRs) and members of the CD94/NKG2 family, Major Histocompatibility Complex (MHC) molecules and help regulate cytotoxic activities of NK cells. In this study, we investigated whether neutrophils have a self-recognition system similar to that of NK cells, focusing on the self-recognition of xenogeneic cells using HLA class Ib and its counter receptor.
The LILR gene family has been studied extensively. ILT-2 and ILT-4 molecules recognize a wide range of HLA class Ia and Ib molecules. Respective ILT-2 and ILT-4 have four and three Immunoreceptor Tyrosine-Based Inhibitory Motifs (ITIM), respectively, in the cytoplasm and transmit inhibitory signals. Many immune cells, including monocytes, macrophages, dendritic cells, B cells, T cells and NK cells, express ILT-2. Monocytes, macrophages and dendritic cells express ILT-4. In addition, the NKG2 family of C-type lectin-like receptors and CD94 form a heterodimer as an NKG2/CD94 molecule that is expressed on NK cells and a subset of T cells [25]. The expression of these molecules on neutrophils has not yet been thoroughly investigated; however, several reports have shown that ILT-2 is expressed at relatively lower and variable levels on neutrophils. In contrast, ILT-4 is highly expressed and localized in the rafts of neutrophils together with CD32a and has been reported to inhibit ROS production and phagocytic functions of neutrophils via stimulation of HLA-G1. These factors are associated with the neutrophil status during inflammation [26,27].
We investigated NKG2A, ILT-2 and ILT-4 expression levels on human neutrophils. ILT-2 and NKG2A were expressed at lower levels, but ILT-4 was relatively well expressed. Characteristically, ILT-2 and ILT-4 bind to the α3 and β2-microglobulin (β2m) domains, which are low polymorphic regions of HLA. In this study, HLA-G1 was transfected into SECs to establish SEC/HLA-G1 cell lines without a hβ2m domain. ILT-4 binds to the α3 domain side of HLA-G1 rather than hβ2m. Therefore, it can bind to HLA-G1 even in the absence of hβ2m. In contrast, ILT-2 is less likely to bind to HLA-G1 in the absence of hβ2m [28-30]. These findings are consistent with those of this study. These findings correlated with expression levels of receptors on neutrophils. Although SEC/HLAE cells showed a tendency to control regulation, which was not significant, the cytotoxicity of SEC/HLA-G1 cells was significantly inhibited. Furthermore, the ROS production measurement test results indicated that HLA-G1 significantly reduced ROS production levels. This is believed to be due to the properties of ILT-4. We investigated the suppression of NETosis by HLA-G1 and HLA-E. Results indicated that HLA-G1 exhibits significant inhibitory effects. Furthermore, significant results were obtained for the suppression of NETosis by HLA-E via the low expression of NKG2A.
Our results suggest that although the suppression of HLA-E in SECs is modest, the expression of HLA-G1 can suppress neutrophil-mediated xenograft immune responses. Additionally, simultaneous transfection of HLA-G1 and hβ2m may enhance the expression level of HLA-G1, making the suppressive effect on neutrophils more effective. We have previously reported this in a study involving NK cells [31]. When developing GE-pigs, we believe that introducing the hβ2m gene simultaneously with HLAG1, rather than HLA-G1 alone, increases HLA-G1 expression. Furthermore, considering that hβ2m can also serve as a ligand site for ILT2/4, it is believed that it would be better to introduce hβ2m simultaneously with HLA-G1.
In conclusion, the expression levels of HLA-G1 in the graft are important, considering the simultaneous regulation of all innate immune cells, neutrophils, macrophages and NK cells in xenografts [32].
While transplants of GE pig hearts and kidneys have begun, pancreatic islet transplantation remains the most common xenotransplantation to date. In pig pancreatic islet transplants, a phenomenon called the Instant Blood-Mediated Inflammatory Reaction (IBMIR) occurs [33,34]. This reaction involves antibodies, a complement system and coagulation factors, followed by the involvement of macrophages and neutrophils. Additionally, pig pancreatic islet transplants are tissue transplants. Rejection reactions in pancreatic islet transplants must occur similarly to those in the heart and kidney transplants. On the other hand, in red blood cell transplantation, is a cellular transplantation and even in this case, not only macrophages but neutrophils as well may also be involved [35-37].
Conclusion
In all clinical trials to date, neutrophil attacks on grafts that do not express HLA-G1/E have not been discussed. We think that there are many factors behind this. This is because xenoantigens such as such as α-Gal and H-D are knocked out and expressed CRPs are suppressing complement deposition. Furthermore, the expression of CD47 controls not only macrophages but neutrophils as well. In addition, large amounts of immunesuppressants, such as metabolic antagonists, calcineurin antagonists and anticomplement agents, are believed to suppress neutrophils. There are various theories regarding the effect of steroids on neutrophils and it is still unknown at present. In the future, we intend to examine the effects of each immune-suppressant on neutrophils both in vitro and in vivo.
These results indicate that HLA-G1 effectively regulates the xenogeneic immune responses of neutrophils against SECs in xenotransplantation.
Acknowledgements
This study was supported by CoMIT Omics Center, Graduate School of Medicine.
Conflicts of Interests
The authors declare that they have no competing financial interests.
References
- Griffith BP, Goerlich CE, Singh AK, Rothblatt M, Lau CL, et al. (2022) Genetically modified porcine-to-human cardiac xenotransplantation. N Engl J Med 387: 35-44.
[Crossref] [Google Scholar] [Indexed]
- Miyagawa S, Maeda A, Toyama C, Kogata S, Okamatsu C, et al. (2022) Aspects of the complement system in new era of xenotransplantation. Front Immunol 13: 860165.
[Crossref] [Google Scholar] [Indexed]
- Ide K, Wang H, Tahara H, Liu J, Wang X, et al. (2007) Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci USA 104: 5062-5066.
[Crossref] [Google Scholar] [Indexed]
- Lu TY, Xu XL, Du XG, Wei JH, Yu JN, et al. (2022) Advances in innate immunity to overcome immune rejection during xenotransplantation. Cells 11: 3865.
[Crossref] [Google Scholar] [Indexed]
- Anand RP, Layer JV, Heja D, Hirose T, Lassiter G, et al. (2023) Design and testing of a humanized porcine donor for xenotransplantation. Nature 622: 393-401.
[Crossref] [Google Scholar] [Indexed]
- Langin M, Mayr T, Reichart B, Michel S, Buchholz S, et al. (2019) Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 564: 430-433.
[Crossref] [Google Scholar] [Indexed]
- Cooper DKC, Pierson RN (2023) Milestones on the path to clinical pig organ xenotransplantation. Am J Transplant 23: 326-335.
[Crossref] [Google Scholar] [Indexed]
- Eisenson D, Hisadome Y, Santillan M, Iwase H, Chen W, et al. (2024) Consistent survival in consecutive cases of life-supporting porcine kidney xenotransplantation using 10GE source pigs. Nat Commun 15: 3361.
[Crossref] [Google Scholar] [Indexed]
- Montgomery RA, Stern JM, Lonze BE, Tatapudi VS, Mangiola M, et al. (2022) Results of two cases of pig-to-human kidney xenotransplantation. N Engl J Med 386: 1889-1898.
[Crossref] [Google Scholar] [Indexed]
- Miyagawa S, Hirose H, Shirakura R, Naka Y, Nakata S, et al. (1988) The mechanism of discordant xenograft rejection. Transplantation 46: 825-830.
[Crossref] [Google Scholar] [Indexed]
- Inverardi L, Samaja M, Motterlini R, Mangili F, Bender JR, et al. (1992) Early recognition of a discordant xenogeneic organ by human circulating lymphocytes. J Immunol 149: 1416-1423.
[Crossref] [Google Scholar] [Indexed]
- Puga Yung G, Schneider MKJ, Seebach JD (2017) The role of NK cells in pig-to-human xenotransplantation. J Immunol Res 2017: 4627384.
[Crossref] [Google Scholar] [Indexed]
- Maeda A, Kogata S, Toyama C, Lo PC, Okamatsu C, et al. (2022) The innate cellular immune response in xenotransplantation. Front Immunol 13: 858604.
[Crossref] [Google Scholar] [Indexed]
- Li S, Yan Y, Lin Y, Bullens DM, Rutgeerts O, et al. (2007) Rapidly induced, T-cell independent xenoantibody production is mediated by marginal zone B cells and requires help from NK cells. Blood 110: 3926-3935.
[Crossref] [Google Scholar] [Indexed]
- Jiaravuthisan P, Maeda A, Takakura C, Wang HT, Sakai R, et al. (2018) A membrane-type Surfactant Protein D (SP-D) suppresses macrophage-mediated cytotoxicity in swine endothelial cells. Transpl Immunol 47: 44-48.
[Crossref] [Google Scholar] [Indexed]
- Iemitsu K, Sakai R, Maeda A, Gadomska K, Kogata S, et al. (2024) The hybrid CL-SP-D molecule has the potential to regulate xenogeneic rejection by human neutrophils more efficiently than CD47. Transpl Immunol 84: 102020.
[Crossref] [Google Scholar] [Indexed]
- Esquivel EL, Maeda A, Eguchi H, Asada M, Sugiyama M, et al. (2015) Suppression of human macrophage-mediated cytotoxicity by transgenic swine endothelial cell expression of HLA-G. Transpl Immunol 32: 109-115.
[Crossref] [Google Scholar] [Indexed]
- Maeda A, Kawamura T, Ueno T, Usui N, Eguchi H, et al. (2013) The suppression of inflammatory macrophage-mediated cytotoxicity and proinflammatory cytokine production by transgenic expression of HLA-E. Transplant Immunology 29: 76-81.
[Crossref] [Google Scholar] [Indexed]
- Miyagawa S, Shirakura R, Iwata K, Nakata S, Matsumiya G, et al. (1994) Effects of transfected complement regulatory proteins, MCP, DAF, and MCP/DAF hybrid, on complement-mediated swine endothelial cell lysis. Transplantation 58: 834-840.
- Matsunami K, Kusama T, Okura E, Shirakura R, Fukuzawa M, et al. (2006) Involvement of position-147 for HLA-E expression. Biochem Biophys Res Commun 347: 692-697.
[Crossref] [Google Scholar] [Indexed]
- Wang HT, Maeda A, Sakai R, Lo PC, Takakura C, et al. (2018) Human CD31 on porcine cells suppress xenogeneic neutrophil-mediated cytotoxicity via the inhibition of NETosis. Xenotransplantation 25: e12396.
[Crossref] [Google Scholar] [Indexed]
- Yoneyama T, Maeda A, Kogata S, Toyama C, Lo PC, et al. (2021) The regulation of neutrophil extracellular trap-induced tissue damage by human Cd177. Transplant Direct 7: e734.
[Crossref] [Google Scholar] [Indexed]
- Miyagawa S, Hirose H, Shirakura R, Naka Y, Nakata S, et al. (1988) The mechanism of discordant xenograft rejection. Transplantation 46: 825-830.
[Crossref] [Google Scholar] [Indexed]
- Galili U, Swanson K (1991) Gene sequences suggest inactivation of alpha-1,3-galactosyltransferase in catarrhines after the divergence of apes from monkeys. Proc Natl Acad Sci USA 88: 7401-7404.
[Crossref] [Google Scholar] [Indexed]
- Ljunggren HG, Karre K (1990) In search of the missing self: MHC molecules and NK cell recognition. Immunol Today 11: 237-244.
[Crossref] [Google Scholar] [Indexed]
- Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, et al. (2003) Human inhibitory receptors Ig-Like Transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA 100: 8856-8861.
[Crossref] [Google Scholar] [Indexed]
- Baudhuin J, Migraine J, Faivre V, Loumagne L, Lukaszewicz AC, et al. (2013) Exocytosis acts as a modulator of the ILT4-mediated inhibition of neutrophil functions. Proc Natl Acad Sci 110: 17957-17962.
[Crossref] [Google Scholar] [Indexed]
- Bankey PE, Banerjee S, Zucchiatti A, De M, Sleem RW, et al. (2010) Cytokine induced expression of programmed death ligands in human neutrophils. Immunol Lett 129: 100-107.
[Crossref] [Google Scholar] [Indexed]
- Allen RL, Raine T, Haude A, Trowsdale J, Wilson MJ (2001) Leukocyte receptor complex-encoded immunomodulatory receptors show differing specificity for alternative HLA-B27 structures. J Immunol 167: 5543-5547.
[Crossref] [Google Scholar] [Indexed]
- Willcox BE, Thomas LM, Chapman TL, Heikema AP, West AP, et al. (2002) Crystal structure of LIR-2 (ILT4) at 1.8 A: Differences from LIR-1 (ILT2) in regions implicated in the binding of the human cytomegalovirus class I MHC homolog UL18. BMC Struct Biol 2: 6.
[Crossref] [Google Scholar] [Indexed]
- Shiroishi M, Kuroki K, Rasubala L, Tsumoto K, Kumagai I, et al. (2006) Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d). Proc Natl Acad Sci USA 103: 16412-16417.
[Crossref] [Google Scholar] [Indexed]
- Matsunami K, Miyagawa S, Nakai R, Murase A, Shirakura R (2001) The possible use of HLA-G1 and G3 in the inhibition of NK cell-mediated swine endothelial cell lysis. Clin Exp Immunol 126: 165-172.
[Crossref] [Google Scholar] [Indexed]
- Rao JS, Hosny N, Kumbha R, Naqvi RA, Singh A, et al. (2021) HLA-G1+expression in GGTA1KO pigs suppresses human and monkey anti-pig T, B and NK cell responses. Front Immunol 12: 730545.
[Crossref] [Google Scholar] [Indexed]
- Hawthorme WJ, Salvaris EJ, Philips P, Hawkes J, Liuwantara D, et al. (2014) Control of IBMIR in neonatal porcine islet xenotransplantation in baboons. Am J Transplant 14: 1300-1309.
[Crossref] [Google Scholar] [Indexed]
- Cooper DKC, Mou L, Bottino R (2024) A brief review of the current status of pig islet xenotransplantation. Front Immunol 15: 1366530.
[Crossref] [Google Scholar] [Indexed]
- Smood B, Hara H, Schoel LJ, Cooper DKC (2019) Genetically-engineered pigs as sources for clinical red blood cell transfusion: What pathobiological barriers need to be overcome? Blood Rev 35: 7-17.
[Crossref] [Google Scholar] [Indexed]
- Park S, Lee H, Park EM, Roh J, Kang PI, et al. (2023) Initial investigation on the feasibility of porcine red blood cells from genetically modified pigs as an alternative to human red blood cells for transfusion. Front Immunol 14: 1298035.
[Crossref] [Google Scholar] [Indexed]
- Gupta AK, Giaglis S, Hasler P, Hahn S (2014) Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One 9: e97088.
[Crossref] [Google Scholar] [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences