Fanconi Syndrome Associated with the use of Tenofovir Treatment in a Patient with Hepatitis B Virus Infection: Reporting a Case
Alejandro Jose Rosales Montero
Department of Nephrology, Regional Hospital of Manacor, Balearic Islands, Spain
Published Date: 2024-02-09DOI10.36648/2472-5056.9.1.230
Rosales Montero Alejandro Jose*, Alvarado Gutierrez Raul and Tura Rosales David
Department of Nephrology, Regional Hospital of Manacor, Balearic Islands, Spain
- *Corresponding Author:
- Alejandro Jose Rosales Montero
Department of Nephrology,
Regional Hospital of Manacor, Balearic Islands,
Spain,
E-mail: alejandrorodriguez25998@gmail.com
Received date: January 09, 2024, Manuscript No. IPJCEN-24-18545; Editor assigned date: January 12, 2024, PreQC No. IPJCEN-24-18545 (PQ); Reviewed date: January 26, 2024, QC No. IPJCEN-24-18545; Revised date: February 02, 2024, Manuscript No. IPJCEN-24-18545 (R); Published date: February 09, 2024, DOI: 10.36648/2472-5056.9.1.230
Citation: Jose RMA, Raul AG, David TR (2024) Fanconi Syndrome Associated with the use of Tenofovir Treatment in a Patient with Hepatitis B Virus Infection: Reporting a Case. J Clin Exp Nephrol Vol.9 No.1: 230.
Abstract
Fanconi syndrome is a rare disorder of the functioning of the renal tubules that results in the excretion of excessive amounts of glucose, bicarbonate, phosphates (phosphorus salts), uric acid, potassium and certain amino acids in the urine, which it can be caused by multiple pathologies, both hereditary and acquired, including those associated with pharmacological nephrotoxicity. Tenofovir Disoproxil Fumarate (TDF) is a nucleotide analogue reverse transcriptase inhibitor used for the treatment of Hepatitis B Virus (HBV) infection, similar in structure to Adefovir and Cidofovir. We present the case of a 63-year-old woman with known HBV infection for 22 years, receiving treatment with Tenofovir. In outpatient check-ups, he reported a progressive picture of asthenia and diffuse bone pain. In several determinations an elevation of alkaline phosphatase and Parathyroid Hormone (PTH) had been observed, in addition to analytical data among which were marked glycosuria, hypophosphatemia and hyperphosphaturia, tubular acidosis with normal GAP anion, hypokalemia and hyperkaliuria, hypouricaemia and hyperuricosuria and subacute deterioration of renal function. All abnormalities resolved after discontinuation of TDF and initiation of treatment for the aforementioned abnormalities, illustrating the importance of clinicians considering the possibility of TDF proximal tubulopathy in patients presenting with clinical manifestations such as polyuria, polydipsia, bone pain, constipation, general syndrome or mineral metabolism abnormalities.
Introduction
Tenofovir Disoproxil Fumarate (TDF), is a nucleotide analogue that blocks the action of reverse transcriptase [1]. Despite its good tolerance and high antiretroviral effectiveness, it can cause cumulative kidney damage. TDF is eliminated through glomerular filtration and proximal tubular secretion. About 20%-30% of the drug is actively transported into the cells of the proximal renal tubule through organic anion transporters located in the basolateral membrane. Subsequently, the drug is secreted into the tubular lumen by apical membrane transporters [2]. Tenofovir Disoproxil Fumarate (TDF) is one of the nucleotide analogue reverse transcriptase inhibitors in the form of esterdisoproxil that is administered once a day, alone, or in combination with other transcontinental drugs [3].
Case Presentation
We present the case of a 63-year-old woman, native of Spain, residing in the municipality of Alcoy, in the province of Alicante, HBV infection (detected through elevated transaminases and surface antigen positivity), known since diagnosed 26 years ago in a hospital in the same town (biopsied in 1997: Chronic hepatitis B), for which he was evaluated by the digestive service, initially starting interferon in December 1997 for one year, with a complete response. Subsequently, in January 2002, he again presented elevated transaminases and a high viral load, starting treatment with lamivudine for a year, without response and subsequently starting tenofovir, presenting a negative viral load after completing a year of treatment, making a decision by the health service. Digestive continuous treatment with said antiviral and remaining a carrier of surface antigen and undetectable viral loads in successive analytical controls. The patient decides to move to the Manacor area in Palma de Mallorca and goes for a check-up at the nephrology service at our hospital. During the last two years, in outpatient check-ups he reported symptoms of asthenia and adynamia associated with loss of appetite, polyuria, nocturia and polydipsia, associated with right coxalgia, diffuse bone pain and finally, for 6 months, symptoms of constipation, which does not improve with the use of laxatives. The patient denies taking nephrotoxic drugs and psychoactive drug use. He does not report toxic habits and comments that he follows hygienic-dietary measures. In the first review, the patient presents a home ambulatory blood pressure record for approximately 2 months until the day of the visit, which reports an average morning BP of 150/95 and nighttime BP of 147/94. Unremarkable physical examination. In two analytical determinations until the visit to our institution, a progressive increase in total Alkaline Phosphatase (ALP), as well as Parathyroid Hormone (PTH) and serum creatinine levels and a progressive decrease in serum phosphorus levels had been observed. Potassium, magnesium, urate, pH and bicarbonate. Serum 25-hydroxyvitamin D, total and ionic calcium, other routine biochemical parameters and urine sediment were normal. No determination of electrolytes, magnesium, phosphorus and proteinuria had been performed in 24-hrs urine.
A new complete analysis is requested from our service, with determination of gases, complete ionogram, venous gases, mineral bone profile, including magnesium levels, in addition to urate, transaminases, FA, viral serology and HBV viral load and a study is requested complete urine analysis (urine analysis and complete biochemical study in 24-hrs urine that included ionogram, calcium, phosphorus, creatinine, urate, glucose, magnesium, total proteins and albumin). Laboratory data included mild hyperglycemia (154 mg/dl), marked glycosuria (4 +), hypophosphatemia, inappropriately high phosphaturia, hypouricemia (2 mg/dl), slight metabolic acidosis (bicarbonate 19 mmol/l) and proteinuria (945 mg). /24h). Other parameters are shown in Annexes Table 1. On the other hand, a bone densitometry, compatible with osteopenia in the total right femur (Image 1 in Annexes). Other secondary causes of FS were ruled out through other analytical studies requested.
Based on these results, the diagnosis of complete Fanconi syndrome with severe hypophosphatemia, nephrogenic diabetes insipidus probably secondary to chronic hypokalemia, chronic kidney disease probably secondary to NTIC due to chronic hypokalemia and probable osteomalacia related to chronic hypophosphatemia, in relation to syndrome, was established. Complete Fanconi syndrome associated with proximal tubule toxicity due to TDF. The case was discussed with the digestive service of our center and it was decided to replace the antiviral treatment (tenofovir was withdrawn and treatment was started with entecavir). In addition, phosphorus supplements were administered in the form of sodium dihydrogen monophosphate in sachets (6 sachets daily), active vitamin D in the form of calcitriol orally (0.25 mcg daily), alkali (sodium bicarbonate, 3 mEq per kg of weight daily) and high-dose oral potassium supplements (6 potassium ascorbic tablets). With this, his condition progressively improved, improving polyuria and polydipsia until its disappearance after 6 months, significant improvement in constipation, improved blood pressure control, demonstrated with the normalization of BP levels in new home ambulatory blood pressure control and bone pain, asthenia and adynamia disappeared, appetite recovered and serum analytical alterations normalized and an undetectable HBV viral load was maintained, although phosphate reabsorption remained discretely low. Control bone densimetry with osteopenia improvement (Image 2 in Annexes).
Results and Discussion
Fanconi syndrome is a well-defined syndrome described as a global dysfunction of the tubule. Proximal due to damage to it, which may be temporary or permanent; the damage may differ in extent and severity from tubular dysfunction leading to urinary excretion of amino acids, glucose, phosphate, bicarbonate and uric acid, leading to acidosis, dehydration and electrolyte disturbance [3].
The causes of Fanconi syndrome, complete or partial, are multiple, with 2 main causes: Congenital, presenting in childhood and acquired, predominantly in adulthood, related to paraproteinemias, tubulointerstitial diseases and drugs or toxins 2. In patients with HBV infection, the most common cause of Fanconi syndrome is the use of drugs, mainly TDF and less frequently other drugs [4-5].
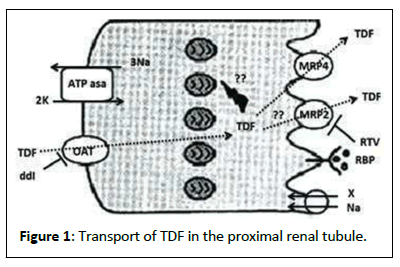
The pathogenic mechanisms involving the effect of TDF suggest that the accumulation of the drug in the proximal tubules would be a key mechanism given its affinity for the Organic Anion Transporter (OAT1 and OAT2) 1 and type 2 and exits the cell through the Multidrug Resistance Protein transporter type 4 (MRP4) and type 2 (OAT2). (MRP2) located in the apical membrane (Figure 1). Recent studies have shown that oxidative stress, inflammation, chronic renal failure, diabetes, malnutrition and the simultaneous prescription of other drugs (Didanosine and protease inhibitors) can facilitate nephrotoxicity [6-7].
Regarding the time of onset of symptoms, some publications have shown that Fanconi syndrome occurs on average within 10 months (1-24 months) after the introduction of TDF.
The clinical manifestations of Fanconi syndrome vary depending on the time of evolution and the degree and type of predominant hydroelectrolyte or metabolic alteration. Patients typically present with polyuria, polydipsia, possibly dehydration, muscle weakness and bone loss (e.g., osteomalacia in adults, growth retardation and rickets in children). The classic constellation of metabolic abnormalities and urinalysis abnormalities with a supporting clinical presentation establishes the diagnosis of the syndrome; the extent of the metabolic disorder and urinary abnormalities depends on the cause of the syndrome and the subsequent extent and degree of proximal tubular dysfunction.
Classic metabolic abnormalities associated with the syndrome include hypophosphatemia, hypokalemia, hypouricemia and hyperchloremic metabolic acidosis with normal anion gap (i.e., type 2 proximal renal tubular acidosis); urinalysis findings include glycosuria, mild proteinuria and aminoaciduria [8].
The treatment is that of the cause that produced it in addition to the correction of associated biochemical alterations: Replacement of alkali to correct acidosis 4 mEq/Kg of HCO3 daily, potassium supplements (potassium citrate, potassium lactate potassium acetate or potassium ascorbate) if hypokalemia and correct hypophosphatemia to prevent progression of bone disease by oral phosphorus supplementation in the form of sodium monophosphate or sodium dihydrogen phosphate salts (intravenous phosphate in the form of potassium monophosphate preferably if severe or symptomatic hypophosphatemia) and supplementation of active vitamin D (calcitriol) [9].
There is also no sensitive and specific marker of renal damage due to TDF, apparently increased phosphate excretion would be the most sensitive marker of tubular dysfunction. In a Swiss study, phosphaturia occurred in 40%-50% of patients with TDF, vs. 25% of patients with other antiretrovirals and 4% of untreated patients [10].
Differential diagnosis
The main differential diagnosis is made based on the clinical history, especially the presence of a family history of hereditary diseases that cause Fanconi syndrome or in the case of acquired diseases that are associated with it.
Prognosis
With adequate treatment, the prognosis of Fanconi syndrome is favorable, especially when it is associated with pharmacological causes, making the general impact on the prognosis of the patient be favorable.
Conclusion
This case illustrates the importance for clinicians to include the possibility of tubular acidosis with normal GAP anion, hypokalemia and hypophosphatemia secondary to proximal tubulopathy in the diagnosis of patients treated with TDF who present with apparently unexplained symptoms such as constipation, polyuria and polydipsia and bone pain, which could on the other hand be attributed to the underlying disease or in our case, to an acquired proximal tubulopathy secondary to direct drug toxicity (tenofovir).
References
- Keefe P, Bokhari SRA (2023) Fanconi Syndrome. Treasure Island (FL): StatPearls Publishing, Florida, United States.
- Cuadrado GB, de los Santos Gil I (2008) Tratamiento de la toxicidad renal en el paciente positivo al virus dela inmunodeficiencia humana. Que medir, como medirlo y con que frecuencia. Enferm Infecc Microbiol Clin 26: 55-61.
[Crossref], [Google Scholar]
- O'Donnell EP, Scarsi KK, Darin KM, Gerzenshtein L, Postelnick MJ, et al. (2011) Low incidence of renal impairment observed in tenofovir-treated patients. J Antimicrob Chemother 66: 1120-1126.
[Crossref], [Google Scholar], [Indexed]
- Gupta SK (2008) Tenofovir-associated Fanconi syndrome: Review of the FDA adverse event reporting system. AIDS Patient Care STDS 22: 99-103.
[Crossref], [Google Scholar], [Indexed]
- Kayaaslan B, Guner R (2017) Adverse effects of oral antiviral therapy in chronic hepatitis B. World J Hepatol 9: 227-241.
[Crossref], [Google Scholar], [Indexed]
- Ramamoorthy H, Abraham P, Isaac B (2014) Preclinical efficacy of melatonin in the amelioration of tenofovir nephrotoxicity by the attenuation of oxidative stress, nitrosative stress and inflammation in rats. J Basic Clin Physiol Pharmacol 27: 1-13.
[Crossref], [Google Scholar], [Indexed]
- Tourret J, Deray G, Isnard-Bagnis C (2013) Tenofovir effect on the kidneys of HIV-infected patients: A double-edged sword? J Am Soc Nephrol 24: 1519-1527.
[Crossref], [Google Scholar], [Indexed]
- Gupta SK, Post FA, Arribas JR, Eron JJ, Wohl DA, et al. (2019) Renal safety of tenofovir alafenamide versus tenofovir disoproxil fumarate: A pooled analysis of 26 clinical trials. AIDS 33: 1455-1465.
[Crossref], [Google Scholar], [Indexed]
- Song K, Yan Q, Yang Y, Lv M, Chen Y, et al. (2020) Fanconi syndrome induced by adefovir dipivoxil: A case report and clinical review. J Int Med Res 48: 300060520954713.
[Crossref], [Google Scholar], [Indexed]
- Hall AM, Edwards SG, Lapsley M, Connolly JO, Chetty K, et al. (2009) Subclinical tubular injury in HIV-infected individual son antiretroviral therapy: A crosssectional analysis. Am J Kidney Dis 54: 1034-1042.
[Crossref], [Google Scholar], [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences