Efficacy and Safety of Denosumab for the Treatment of Osteoporosis in Patients with Chronic Kidney Disease
Hitoshi Suzuki, Masao Kihara, Satoshi Mano, Takashi Kobayashi, Yasuhiko Kanaguchi, Teruo Hidaka, Tomohito Gohda and Yusuke Suzuki
DOI10.21767/2472-5056.100030
Hitoshi Suzuki, Masao Kihara, Satoshi Mano, Takashi Kobayashi, Yasuhiko Kanaguchi, Teruo Hidaka, Tomohito Goda and Yusuke Suzuki*
Department of Nephrology, Faculty of Medicine, Juntendo University, Tokyo, Japan
- *Corresponding Author:
- Yusuke Suzuki
Department of Nephrology, Faculty of Medicine
Juntendo University, Tokyo, Japan
Tel: +81-3-5802-1064
E-mail: yusuke@juntendo.ac.jp
Received date: March 23, 2017; Accepted date: March 27, 2017; Published date: March 30, 2017
Citation: Suzuki H, Kihara M, Mano S, Kobayashi T, Kanaguchi Y, et al. (2017) Efficacy and Safety of Denosumab for the Treatment of Osteoporosis in Patients with Chronic Kidney Disease. J Clin Exp Nephrol 2:30. doi: 10.21767/2472-5056.100030
Copyright: © 2017 Suzuki H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Chronic kidney disease (CKD) is an independent risk factor for osteoporosis and may lead to metabolic abnormalities that accelerate bone loss. Bisphosphonates, the most widely used treatment for osteoporosis, are contraindicated in patients with severe renal impairment. In the present study, we assessed whether denosumab is a safe and effective treatment for osteoporosis in patients with CKD.
Methods and Findings: A total of 143 patients with osteoporosis who were treated with denosumab were analyzed retrospectively. Of these patients, 40 had been previously treated with bisphosphonates. All patients received supplemental vitamin D. Effectiveness was assessed by analyzing changes in bone mineral density (BMD) and serum tartrate-resistant acid phosphatase (TRACP)-5b as a marker for serum bone resorption. More than fifty percent of the patients treated with bisphosphonates showed low BMD at the time their therapy was changed to denosumab. Denosumab was associated with a larger increase in both lumbar and femur neck BMD than were bisphosphonates (+4.8% and +5.5%, respectively). Denosumab decreased serum TRACP-5b while increasing BMD (P<0.001), and was well tolerated. Serum calcium levels decreased shortly after the injection of denosumab, but recovered within 14 days. Supplemental vitamin D (0.5 to 1.0 μg/day) appeared to prevent hypocalcemia and to support efficacy of denosumab.
Conclusions: Denosumab increases BMD in the lumbar vertebra and femur neck in patients with CKD. The effect of denosumab on BMD is greater than that of bisphosphonates in these patients.
Keywords
Denosumab; Bisphosphonates; Osteoporosis; CKD; Corticosteroid
Abbreviations
BMD: Bone Mineral Density; CKD: Chronic Kidney Disease; GFR: Glomerular Filtration Rate; RANK: Receptor Activator of Nuclear Factor-Kappa B; RANKL: Receptor Activator of Nuclear Factor-Kappa B ligand; TRACP-5b: Serum Tartrate-Resistant Acid Phosphatase 5b; YAM: Young Adult Mean.
Introduction
Osteoporosis, which is characterized by low bone mineral density (BMD) and microarchitectural deterioration of bone tissue, is associated with an increased risk of fracture [1]. Bone loss can take place as a result of estrogen deficiency in postmenopausal women, and the risk of fracture correlates with increasing age and decreasing BMD [2].
The incidence of chronic kidney disease (CKD) also increases with age. More than half of individuals older than 70 years have a decreased estimated glomerular filtration rate (eGFR) (<60 mL/min/1.73 m2) [3]. CKD is an independent risk factor for osteoporosis [4-7]. Individuals with CKD may have an increased risk for osteoporosis for several reasons, including shared risk factors for both conditions such as advanced age and female gender. In addition, CKD may lead to metabolic abnormalities that accelerate bone loss, such as chronic metabolic acidosis, hypogonadism, hyperparathyroidism, and abnormalities of vitamin D metabolism [8]. Osteoporosis can also be induced by glucocorticosteroids, which are widely used as an immunosuppressive agent for glomerulonephritis. Bone loss is a serious side effect of this therapy [9].
Bisphosphonates are the most widely used treatment for osteoporosis. They reduce the risk of fracture in the short term. The critical problem is that there is no evidence of efficacy for long-term treatment [10,11]. Moreover, bisphosphonates may lead to a deterioration of renal function [12]. Thus, bisphosphonates are either not recommended, or contraindicated outright, for use in patients with severe renal impairment [12,13].
The receptor activator of nuclear factor-kappa B ligand (RANKL) is essential in the formation, activation, and survival of osteoclasts [14]. Denosumab binds RANKL with high affinity and specificity, preventing activation of its cognate receptor RANK, which is expressed on the surface of osteoclasts and osteoclast precursors. Inhibition of Signaling through the RANK receptor prevents osteoclast maturation, activation, and survival, thereby decreasing resorption of cortical and trabecular bone [15]. Inhibition of bone resorption with denosumab improves the structural strength of bone [16]. Treatment with denosumab has been associated with significant reductions in fracture risk across a wide range of patient groups [15,17]. Furthermore, long-term clinical trial follow-up data from the FREEDOM study demonstrates that denosumab treatment for up to 8 years is associated with a persistent reduction of bone turnover, continued increases in BMD without therapeutic plateau, and low fracture incidence [18]. It is unknown whether treatment for osteoporosis with denosumab is safe or effective in patients with CKD. In the present study, we examined the efficacy and adverse effects of denosumab treatment in patients with CKD in real-world clinical practice.
Methods
Participants
A total of 143 patients with CKD who had been treated with denosumab were analyzed retrospectively. There were no exclusion criteria based on renal function. However, the patients were excluded if they had not received a second injection within 7 months of the first; this time window (6 months plus a 1- month grace period) represents the coverage period of a denosumab injection. During the study, all the patients concomitantly received vitamin D. This study was approved by the relevant institutional review boards. All patients provided informed consent to allow access to their relevant medical records.
BMD measurement
BMD was measured by dual-energy X-ray absorptiometry (DEXA) at the spine and femur neck before initiation of denosumab and every 6 months thereafter. BMD values were expressed as percent young adult mean (YAM).
Evaluations of clinical data
Patients underwent regular physical examinations, hematological monitoring, blood chemistry measurements, and urine analysis. We included patients whose serum creatinine, albumin-adjusted calcium, phosphate, and alkaline phosphatase had been measured every 3 months. Serum tartrate resistant acid phosphatase (TRACP)-5b was measured every 6 months. Serum TRACP-5b is used as a marker for bone resorption because it is derived from bone-resorbing osteoclasts, and has the advantage of not being influenced by renal dysfunction [19]. All measurements were performed centrally in a single batch at our hospital and at a validated institution (SRL, Tokyo, Japan).
Statistical Analyse
Paired t-tests were used to compare YAM values recorded at baseline with those recorded after every 6 months of treatment with denosumab. Percent changes from baseline in YAM serum markers at each time point were compared with baseline measurements using ANOVA. A significance level of 0.05 was used. All statistical analyses were performed using GraphPad Prism statistical software (Version 5, GraphPad Software, San Diego, CA, USA).
Results
Baseline characteristics
Characteristics of the patients are summarized in Table 1. A total of 143 patients with osteoporosis were enrolled in this study. Patients with each stage of CKD (based on estimated GFR) were included, including hemodialysis patients. Ninety-five patients had received denosumab as the first therapy for osteoporosis, and 40 had received bisphosphonates before their therapy was changed to denosumab.
| Gender | Male 44, Female 99 | |
| Age | 19 - 96 years (mean: 65.2 years) | |
| CKD stage | non-CKD | 40 |
| Stage 2 | 20 | |
| Stage 3a+3b | 46 | |
| Stage 4 | 23 | |
| Stage 5 | 8 | |
| Stage 5D | 6 | |
| Medication | Denosumab: initial therapy | 95 |
| exchange from vitaminD | 6 | |
| exchange from SERM | 2 | |
| exchange from Bis | 40 |
Table 1: Baseline characteristics. SERM: Selective Estrogen Receptor Modulator; Bis: Bisphosphonates.
Osteoporosis was not satisfactorily treated with bisphosphonates
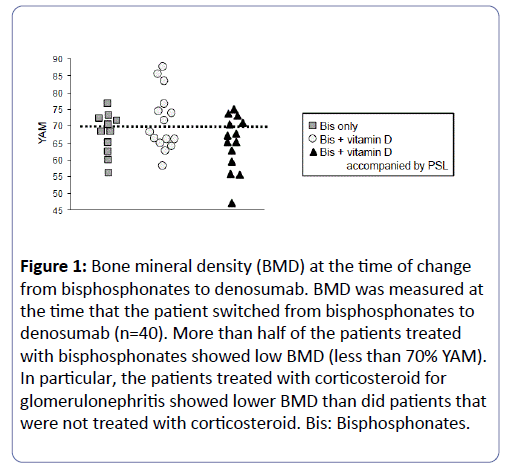
At the time of change from bisphosphonates to denosumab, BMD was measured in each subject (n=40). Surprisingly, more than half of the patients treated with bisphosphonates showed low BMD (less than 70% YAM; Figure 1). In particular, patients treated with a corticosteroid for glomerulonephritis showed low BMD despite bisphosphonate therapy.
Figure 1: Bone mineral density (BMD) at the time of change from bisphosphonates to denosumab. BMD was measured at the time that the patient switched from bisphosphonates to denosumab (n=40). More than half of the patients treated with bisphosphonates showed low BMD (less than 70% YAM). In particular, the patients treated with corticosteroid for glomerulonephritis showed lower BMD than did patients that were not treated with corticosteroid. Bis: Bisphosphonates.
Denosumab increased BMD more than bisphosphonates did
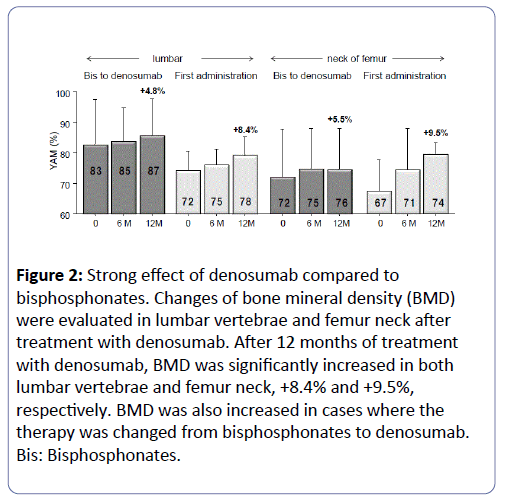
Changes in BMD were evaluated in the lumbar vertebrae and femur neck after treatment with denosumab. After 12 months of treatment with denosumab, BMD increased in lumbar vertebra and femur neck by +8.4% and +9.5%, respectively (Figure 2). BMD also increased in cases where the therapy was changed from bisphosphonates to denosumab, with the increase in lumbar vertebra and femur neck being +4.8% and +5.5%, respectively (Figure 2).
Figure 2: Strong effect of denosumab compared to bisphosphonates. Changes of bone mineral density (BMD) were evaluated in lumbar vertebrae and femur neck after treatment with denosumab. After 12 months of treatment with denosumab, BMD was significantly increased in both lumbar vertebrae and femur neck, +8.4% and +9.5%, respectively. BMD was also increased in cases where the therapy was changed from bisphosphonates to denosumab. Bis: Bisphosphonates.
Denosumab treatment improved bone metabolic markers and was accompanied by persistent increase in BMD
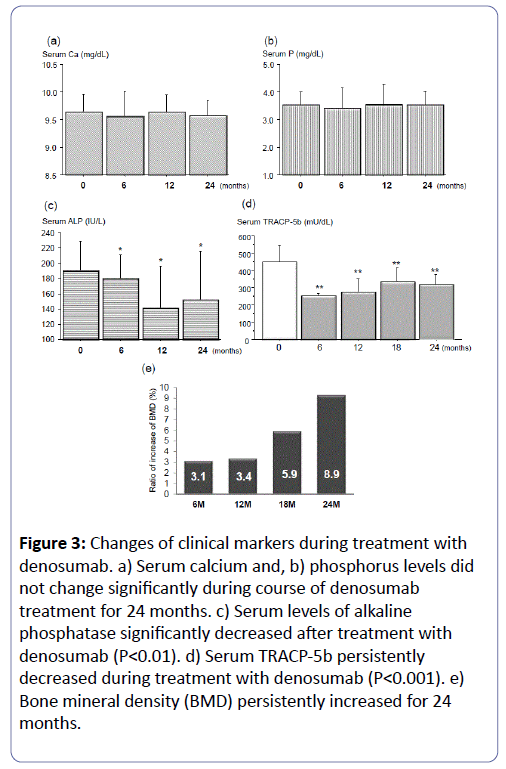
Clinically relevant changes were not seen in serum chemistry, hematology, or urine analyses. No adverse events were reported. During the course of denosumab treatment for 24 months, serum calcium and phosphorus levels did not change significantly (Figures 3a and b). Serum levels of alkaline phosphatase significantly decreased after treatment with denosumab (P<0.01, Figure 3c). Moreover, serum TRACP-5b persistently decreased during treatment with denosumab (P<0.001, Figure 3d). Of note, BMD continuously increased for 24 months (Figure 3e).
Figure 3: Changes of clinical markers during treatment with denosumab. a) Serum calcium and, b) phosphorus levels did not change significantly during course of denosumab treatment for 24 months. c) Serum levels of alkaline phosphatase significantly decreased after treatment with denosumab (P<0.01). d) Serum TRACP-5b persistently decreased during treatment with denosumab (P<0.001). e) Bone mineral density (BMD) persistently increased for 24 months.
Changes in serum calcium after injection of denosumab
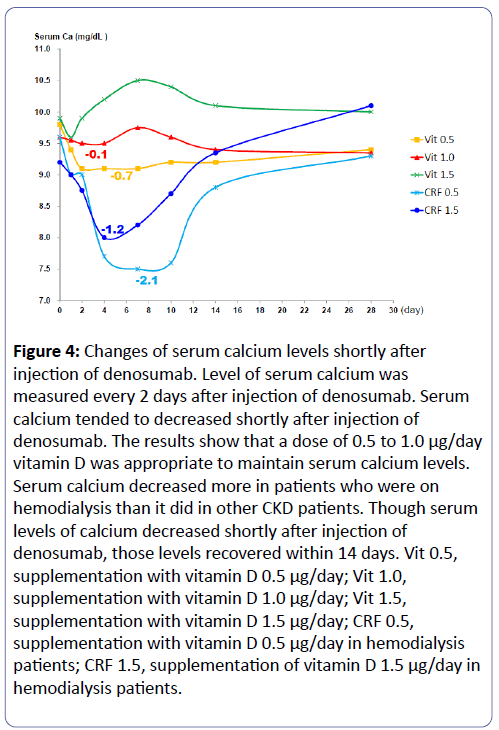
The level of serum calcium was measured every 2 days after injection of denosumab. Serum calcium tended to decrease shortly after injection of denosumab (Figure 4). The decreases in serum calcium differed depending on the dose of vitamin D supplementation. A vitamin D dose of 0.5 to 1.0 μg/day was associated with maintenance of low serum calcium levels (Figure 4). The serum calcium of patients who were on hemodialysis decreased more than that of other CKD patients. Importantly, levels of serum calcium in all patients recovered after 14 days, even though serum calcium decreased shortly after injection of denosumab.
Figure 4: Changes of serum calcium levels shortly after injection of denosumab. Level of serum calcium was measured every 2 days after injection of denosumab. Serum calcium tended to decreased shortly after injection of denosumab. The results show that a dose of 0.5 to 1.0 μg/day vitamin D was appropriate to maintain serum calcium levels. Serum calcium decreased more in patients who were on hemodialysis than it did in other CKD patients. Though serum levels of calcium decreased shortly after injection of denosumab, those levels recovered within 14 days. Vit 0.5, supplementation with vitamin D 0.5 μg/day; Vit 1.0, supplementation with vitamin D 1.0 μg/day; Vit 1.5, supplementation with vitamin D 1.5 μg/day; CRF 0.5, supplementation with vitamin D 0.5 μg/day in hemodialysis patients; CRF 1.5, supplementation of vitamin D 1.5 μg/day in hemodialysis patients.
Discussion
Impaired renal function is a risk factor for osteoporosis, progressive bone loss, and ultimately, bone fractures. Several factors could explain the association between CKD and osteoporosis and osteopenia. Patients with CKD are likely to be older and have lower levels of vitamin D. In addition, there is increasing evidence that CKD itself is a risk factor for low BMD [4-7]. Patients with impaired renal function have been found to have greater rates of bone loss [7,20]. Nickolas et al. reported an independent correlation between an eGFR <60 mL/min/1.73 m2 and the prevalence of hip fractures [21]. In addition, elevated serum cystatin C levels have been independently associated with risk for hip fracture [22,23].
Bisphosphonates are commonly prescribed to treat osteoporosis. However, several studies indicate that bisphosphonates are not efficacious for long-term improvement in BMD [10,11]. Moreover, several studies show poor medication adherence with bisphosphonates [24,25]. Nonadherence with oral bisphosphonates can be associated with significantly increased short-term risk of osteoporotic fractures. In fact, the present study clearly showed that more than half of patients with CKD treated with bisphosphonates had low BMD (<70% YAM) (Figure 1). In particular, patients treated with a corticosteroid for glomerulonephritis had low BMD despite bisphosphonate therapy. Importantly, BMD significantly increased in cases where the therapy was changed from bisphosphonates to denosumab (Figure 2). Denosumab is a potent antiresorptive with antifracture efficacy comparable to that of bisphosphonates. Bisphosphonates affect mature osteoclasts, whereas denosumab prevents osteoclast maturation, activation, and survival, thereby decreasing resorption of cortical and trabecular bone [15]. This may explain why denosumab appears to be more effective than bisphosphonates as a treatment for low BMD.
We also assessed the adverse effects of denosumab in patients with CKD. Although hypocalcemia was a concern, none of the participants showed symptomatic hypocalcemia (Figure 3a). Our results suggest that vitamin D supplementation is required to prevent hypocalcemia, and ideally should be initiated prior to treatment with denosumab because of the decrease of serum calcium that occurs shortly after the injection of denosumab (Figure 4). Higher amounts of vitamin D supplementation are required prior to use of denosumab for patients on hemodialysis. However, levels of serum calcium recovered within 14 days in each case. Moreover, vitamin D supplementation is important to elicit the effect of denosumab. A dose of 0.5 to 1.0 μg/day of vitamin D was required to elicit the effect of denosumab (data not shown). The frequent surveillance of serum and possibly urine calcium are demanded.
Irrespective of cause, patients with CKD have a greater prevalence of osteoporosis and are at increased risk for bone fractures. Despite this increased risk, the majority of studies evaluating osteoporosis therapy have attempted to exclude patients with CKD. The results of the present study suggest that denosumab is an effective intervention for osteoporosis management in patients with CKD. Impaired renal function does not affect the pharmacokinetics and pharmacodynamics of denosumab [26]. After administration of denosumab, serum levels of calcium decreased, as is expected with antiresorptive therapy. The serum calcium kinetics following administration of denosumab (Figure 4) provide evidence for the importance of adequate vitamin D supplementation in real-world clinical practice.
References
- Boyanov M, Shinkov A, Psachoulia E, Intorcia M, Petkova R (2017) Baseline Characteristics and Changes in Bone Mineral Density T-Scores of Bulgarian Women with Postmenopausal Osteoporosis Receiving Denosumab in Routine Clinical Practice. Drugs RD 17: 125-132.
- Kanis JA, Johnell O, Oden A (2001) Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int 12: 989-995.
- Jassal SK, von Muhlen D, Barrett-Connor E (2007) Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: the Rancho Bernardo study. J Bone Miner Res 22: 203-210.
- Buchanan JR, Myers CA, Greer RB 3rd (1988) Effect of declining renal function on bone density in aging women. See comment in PubMed Commons below Calcif Tissue Int 43: 1-6.
- Yendt ER, Cohanim M, Jarzylo S, Jones G, Rosenberg G (1991) Bone mass is related to creatinine clearance in normal elderly women. See comment in PubMed Commons below J Bone Miner Res 6: 1043-1050.
- Lindberg JS, Moe SM (1999) Osteoporosis in end-state renal disease. See comment in PubMed Commons below Semin Nephrol 19: 115-122.
- Nickolas TL, Leonard MB, Shane E (2008) Chronic kidney disease and bone fracture: a growing concern. See comment in PubMed Commons below Kidney Int 74: 721-731.
- Pitts TO, Piraino BH, Mitro R, Chen TC, Segre GV, et al. (1988) Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab 67: 876-881.
- van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. See comment in PubMed Commons below Osteoporos Int 13: 777-787.
- Nakamura T, Nakano T, Ito M, Hagino H, Hashimoto J, et al. (2013) Clinical efficacy on fracture risk and safety of 0.5 mg or 1 mg/month intravenous ibandronate versus 2.5 mg/day oral risedronate in patients with primary osteoporosis. Calcif Tissue Int 93: 137-146.
- Reid IR (2015) Short-term and long-term effects of osteoporosis therapies. See comment in PubMed Commons below Nat Rev Endocrinol 11: 418-428.
- Perazella MA, Markowitz GS (2008) Bisphosphonate nephrotoxicity. See comment in PubMed Commons below Kidney Int 74: 1385-1393.
- Toussaint ND, Elder GJ, Kerr PG (2009) Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol 4: 221-233.
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, et al. (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 31: 3597-3602.
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, et al. (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. See comment in PubMed Commons below N Engl J Med 361: 756-765.
- Ominsky MS, Stouch B, Schroeder J (2011) Denosumab, a fully human RANKL antibody, reduced bone turnover markers and increased trabecular and cortical bone mass, density, and strength in ovariectomized cynomolgus monkeys. Bone 49: 162-173.
- Boonen S, Adachi JD, Man Z, Cummings SR, Lippuner K, et al. (2011) Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. See comment in PubMed Commons below J Clin Endocrinol Metab 96: 1727-1736.
- Papapoulos S, Lippuner K, Roux C, Lin CJ, Kendler DL, et al. (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int 26: 2773-2783.
- Yamada S, Inaba M, Kurajoh M, Shidara K, Imanishi Y, et al. (2008) Utility of serum tartrate-resistant acid phosphatase (TRACP5b) as a bone resorption marker in patients with chronic kidney disease: independence from renal dysfunction. Clin Endocrinol 69: 189-196.
- Bianchi ML, Colantonio G, Montesano A, Trevisan C, Ortolani S, et al. (1992) Bone mass status in different degrees of chronic renal failure. See comment in PubMed Commons below Bone 13: 225-228.
- Nickolas TL, Stein EM, Dworakowski E, Nishiyama KK, Komandah-Kosseh M, et al. (2013) Rapid cortical bone loss in patients with chronic kidney disease. See comment in PubMed Commons below J Bone Miner Res 28: 1811-1820.
- Jassal SK, von Muhlen D, Barrett-Connor E (2007) Measures of renal function BMD, bone loss, and osteoporotic fracture in older adults: The Rancho Bernardo Study. J Bone Miner Res 22: 203-210.
- Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, et al. (2007) Association of kidney function with incident hip fracture in older adults. See comment in PubMed Commons below J Am Soc Nephrol 18: 282-286.
- Sharman Moser S, Yu J, Goldshtein I, Ish-Shalom S, Rouach V, et al. (2016) Cost and Consequences of Nonadherence With Oral Bisphosphonate Therapy: Findings From a Real-World Data Analysis. See comment in PubMed Commons below Ann Pharmacother 50: 262-269.
- Wu X, Wei D, Sun B, Wu XN (2016) Poor medication adherence to bisphosphonates and high self-perception of aging in elderly female patients with osteoporosis. See comment in PubMed Commons below Osteoporos Int 27: 3083-3090.
- Block GA, Bone HG, Fang L, Lee E, Padhi D (2012) A single-dose study of denosumab in patients with various degrees of renal impairment. See comment in PubMed Commons below J Bone Miner Res 27: 1471-1479.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences