Dent disease: About 4 Adult Observations Monitored in the Department of Nephrology and Organ Transplantation of Toulouse, France
Gueye Serigne, Kane Yaya, Seck Sidy Mouhamed, Chauveau Dominique, Lemrabott Ahmed Tall, Faye Maria, Cisse Mouhamadou Moustapha, Diallo Kalilou, Ka El Fary, Niang Abdou, Diouf Boucar.
DOI10.21767/2472-5056.100017
Gueye Serigne1, Kane Yaya2*, Seck Sidy Mouhamed3, Chauveau Dominique1, Lemrabott Ahmed Tall4, Faye Maria4, Cisse Mouhamadou Moustapha4, Diallo Kalilou2, Ka El Fary3, Niang Abdou4 And Diouf Boucar4.
1Nephrology and Transplant Department Hospital of Toulouse, France
2Nephrology and Internal Medicine Department of Assane Seck University, Ziguinchor, Senegal
3Nephrology Department of Gaston Berger University, Saint-Louis, Senegal
4Nephrology Department of Teaching Hospital Aristide Le Dantec, Dakar, Senegal
- *Corresponding Author:
- Yaya Kane
MD, Assane Seck University
Ziguinchor, Senegal
Tel: 0022177 500 21 65
Email: yayuskanus@yahoo.fr
Received date: June 16, 2016; Accepted date: August 08, 2016; Published date: August 11, 2016
Citation: Serigne G, Yaya K, Mouhamed SS, Dominique C, Tall LA, et al. (2016) Dent disease: About 4 Adult Observations Monitored in the Department of Nephrology and Organ Transplantation of Toulouse, France. J Clin Exp Nephrol 1:17. DOI: 10.21767/2472-5056.100017
Copyright: © 2016 Serigne G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: The Dent's Disease (Dd) Is An X-Linked Genetic Kidney Disease. We Undertook A Retrospective Study Of 4 Dd Observations Monitored In The Department Of Nephrology And Transplantation In Toulouse To Relate The History Of The Disease. Our Purpose Was To Show The Phenotypic Variability And Therapeutic Difficulties.
Case Report
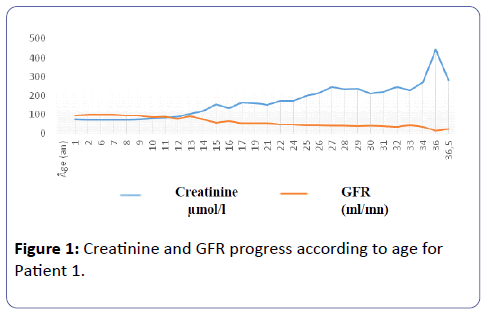
Patient 1: Mr. G.O. - The Dd Was Discovered Incidentally Through Proteinuria Of 0.2 G/24 Hours Associated With Polyuria And Polydipsia Syndrome With Impaired Urine Concentrating And Diluting Capacity. A Minus 1 Standard Deviation Was Noted In One Year In Size And Weight, Which Lasted Up To 8 Years. At 7 Years Of Age, Hypercalciuria Of 8.4 Mg/Kg/Day With Nephrocalcinosis Appeared. From 16 Years Onward, Creatinine Degradation Gradually Reached A Peak Of 444 µmol/L Or A Glomerular Filtration Rate (Gfr), Under The Mdrd Formula, At 22 Ml /Minute In June 2014 In The Occurrence Of Epiglottitis. A Point Mutation Of The Chloride Channel Gene (Cncl5) Was Noted.
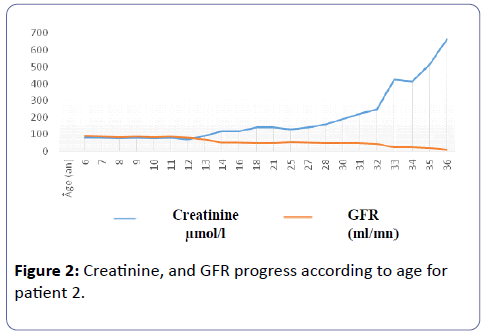
Patient 2: Mr. B.R. The Dd Finding Was Incidental To Proteinuria Of 2.6 G/24 Hours Associated With Polydipsia-Polyuria During A School Visit At The Age Of 6 Without Change In Urinary Diluting Concentration. There Was An Episode Of Renal Colic When He Was 8 Years Old. When He Was 10, Hypercalciuria Of 9 Mg/Kg/Day Was Noticed, Nephrocalcinosis Too. From 14 Years Onward, Creatinine Gradually Deteriorated, Bringing Mr. B. R To Hemodialysis. Genetic Analysis Showed Adenine Deletion At Position 1751, Exon 10.
Patient 3: Mr. G.C. The Discovery Of His Kidney Disease Was Fortuitous As Proteinuria Was Detected In Preventive Medicine When He Was 19 Years Old. There Was No Clinical Manifestation. His Gfr Altered To Average At 58 Ml / Min Using Mdrd Formula. Mutation Of Gene Cic.5 Was Noted. There Was Nephrocalcinosis Associated With Renal Cysts.
Patient 4: Mr. T.C. Is The Younger Brother Of Mr. G.C. The Genetic Investigation Carried Out In The Family Showed That He Was Bearing The Same Mutation As His Elder Brother. He Had No Symptoms, At Most Proteinuria Of 0.5 G/G With Urinary Calcium/Creatinine Concentration Of 0.6. At The Age 20, His Kidneys Worked Normally.
Conclusion: The Therapeutic Resources Were Limited. Several Questions Remained Unanswered, Namely The Impact Of Ace Inhibitors And Arbs In Slowing The Ckd; Also 25-35% Of Cases Of Dd Without Genetic Mutation Was Observed.
https://maviyolculuk.online/
https://mavitur.online/
https://marmaristeknekirala.com.tr
https://tekneturumarmaris.com.tr
https://bodrumteknekirala.com.tr
https://gocekteknekirala.com.tr
https://fethiyeteknekirala.com.tr
Keywords
Polyuria-polydipsia; RBP; β2 microglobulin urine; urinary calcium; Nephrocalcinosis; IRC; Age
Introduction
The Dent's disease (DD) is an X-linked genetic kidney disease related to a major impairment in the proximal convoluted tubule (PCT). It is an inherited monogenic kidney stone disease, among nephrolithiasis cases. These are relatively rare, accounting for about 2% of urinary stones in adults, and 10% in children [1]. The DD comprises three familial syndromes characterized by hypercalciuric nephrolithiasis and X-linked transmission: X-linked recessive nephrolithiasis, hypophosphatemic rickets, connected to familial idiopathic low-molecular-weight proteinuria observed in Japanese patients. [2] The prevalence of DD is unknown, about 250 families have been identified worldwide [3,4]. Before the genetic cause of DD was identified, authentic DD and a moderate phenotype of the Lowe syndrome were misinterpreted as Dent disease. The first results from a mutation of the CLCN5 gene which is located on the short arm of the X chromosome (Xp11.22 more precisely) and encoding the CIC-5. The second results from a mutation of the OCRL1 gene at Xq26.1 which encodes phosphoinositide enzyme [5,6], bisphosphate (PIP2) 5-phosphatase and resulting in oculocerebrorenal syndrome or Lowe syndrome. It is important to note that approximately 25 to 35% of patients with Dent's disease have none of the above-described mutations [7]. This leaves open the hypothesis that other unidentified genes should be involved. We undertook a retrospective study of 4 Dent disease observations monitored in the Department of Nephrology and Transplantation in Toulouse to clarify the clinical, biological, morphological and genetic diagnoses; the therapeutic and evolutionary aspects too. Our purpose was to show the phenotypic variability and therapeutic difficulties.
Case Historie
Patient 1: Mr. G.O, is only son. The DD was discovered incidentally to proteinuria of 0.2 g/24 hours during the neonatal period. This continued throughout the first year of life associated with a polyuria-polydipsia syndrome estimated at 1 l/24 hours at one year of age, 1-2 l during childhood and 5 to 6l in adulthood. Net impaired urinary diluting concentration was noted in the first year of life by testing the concentration of urine using DDAVP. The maximum urinary osmolarity was 116 mosm/l. There was no renal colic. There was no weight retardation though a standard deviation (SD) of –1 was observed at the age of one year in terms of size and weight; such failure continued until aged 8. There was no bone deformity either. GFR remained normal until the age of 15. From 16 years onward, creatinine level gradually altered to a peak of 444 μmol/l or GFR of 22 mL / minute, according to MDRD formula, in June 2014 on the occurrence of epiglottitis, then back to lowest level, 283 μmol/l, after the infectious episode (Figure 1).
Serum levels of phosphate, calcium and uric acid were normal. Vitamin D deficiency (25 OH D3) substituted was noted at 24 years old. At the age of 30, parathyroid hormone rose to 1.5 times the normal level. Blood electrolytes were often normal, sometimes with metabolic alkalosis and hypokalemia during the period of treatment with thiazide diuretics. Proteinuria was both glomerular and tubular. Initially low around 0.2 g/24 hours, it increased gradually during childhood to a peak of 2.7 g/24 hours at the age of 13. Creatinine concentration was 1.07 ± 0.48 g/g on average at adulthood (Table 1).
| Patients | Average proteinuria/Creatinine (g/g) | Average micro albuminuria (mg/day) | Urinary β2-microglobulin (mg/day) | RBP g/l (g/day) | Average 24 h-Calciuria (mg/kg/days) | Calciuria/creatinuria |
|---|---|---|---|---|---|---|
| nº 1 | 1.07 ± 0.48 | 497.55 ± 276.55 | 258 | 2.2 | 9.27 ± 7.02 | - |
| nº 2 | 1.25 ± 0.88 | - | 112 | 1.855 | 5.72 ± 2.19 | - |
| nº 3 | 1.81 ± 0.41 | 192.375 ± 38.7 | 52.95 ± 30.33 | - | - | 0.6 |
| nº 4 | 0.45 ± 0.05 | 195 | - | - | - | 0.5 |
Table 1: Biochemical urinary parameters of 4 patients; RBP: Retinol banding protein, TRP: Tubular reabsorption of phosphate, ER: Excretion rate.
At the age of 7 years, hypercalciuria at 185 mg/24 h or 8.4/kg/day appeared for the first time, with urinary calcium/ creatinine ratio rising from 1.12 to 5.2 under fasting conditions while nephrocalcinosis lesions were noted. There was no amino acids in the urine. Bone densitometry carried out during adulthood showed a slight deficit of 7% with a minimal risk of fracture. T-score of - 1.5 was the last tangible event in 2012. A point mutation in the gene encoding the chloride channel (CNCL5) was noted in 2002. Such change was also seen in his mother. From 7-11 years old, he was given thiazide diuretics. This therapy was stopped at 11 for treatment failure marked by persistent urinary calcium despite optimal dose. Adalat Retard was used for a year and then stopped as the result of inefficiency. Thiazide diuretic was renewed from 13 to 15 years of age, then stopped due to signs of hemoconcentrations. Indomethacin was applied for a year and then stopped because of signs of digestive intolerance.
From 17 to 26, Mr. G.O. was under no treatment. An antiproteinuric nephroprotective treatment with IEC and ARAII (ACE inhibitors and angiotensin II receptor antagonists) was conducted and then stopped due to signs of digestive intolerance.
Patient 2: Mr. B. R, his maternal grandfather died of kidney failure. The discovery of the DD was incidental to proteinuria of 2.6 g/24 hours associated with polyuria-polydipsia of 2 to 5 l during a school visit at the age of 6. The urine concentration was not altered when the urine concentration test was carried out with DDAVP because urinary osmolality reached a peak at 637 mOsm/l. In adulthood, muscle cramps and physical fatigue started associated with sometimes severe persistent hypokalemia, hypomagnesemia, and hypocalcemia (Table 2).
| Average serum calcium in mmol/L | Average serum phosphate in mmol/L | Average alkaline phosphatase (AP) level | Average PTHi | Average 25 (OH) Vit D3 | Average blood sodium | Average serum potassium | Average alkaline reserve (AR) | |
|---|---|---|---|---|---|---|---|---|
| Patient 2 | 2.29 ± 0.25 | 1.06 ± 0.32 | 391.91 ± 183.39 | 306.28 ± 213.23 | 33.33 ± 18.55 | 140.18 ± 2.86 | 3.29 ± 0.66 | 25.2 ± 3.64 |
Table 2: Biochemical blood parameters of Mr. B. R (patient 2); iPTH: Intact Parathyroid Hormone; APL : Alkaline Phosphatase Level; AR : Alkaline Reserve.
Proteinuria was exclusively tubular, 1.25 ± 0.88 g/l on average (Table 1). When he was 10, hypercalciuria at 290 mg/24 h, or 9 mg/kg/day was noted for the first time, with urinary calcium/creatinine ratio which rose from 0.77 to 1; nephrocalcinosis lesions were apparent at the same time. Genetic confirmation of the DD was done in 2004 evidenced by adenine deletion at 1751 in exon 10 causing a shift in the reading frame from the codon 584 and introducing a stop codon at 585. This mutation was also observed at the heterozygous state in his mother.
He had an episode of renal colic at the age of 8. There was no weight retardation nor bone deformity. GFR remained normal until he was 12. From 14 years onward, creatinine concentration deteriorated gradually, accelerating from the age of 32 and drove Mr. B. R to hemodialysis for lack of family transplant (Figure 2).
Treatment with thiazide diuretic was started at the age of 10 years following the onset of urinary calcium and nephrocalcinosis. The clinical and biological tolerance was very bad with vomiting, intense physical asthenia with alkalosis and acute hypokalemia at 1.8. The treatment was stopped three days after it was started. Indomethacin was introduced for one year and then stopped despite its efficacy in urinary calcium (ranging from 6.6 to 2 mg/kg/day) with signs of digestive intolerance. A new attempt to reintroduce thiazide in low dose resulted in a gross early failure, an alkalosis associated with symptomatic hypokalemia. A treatment with a calcium channel blocker (Adalat Retard) was conducted for a year and then stopped due to inefficiency on urinary calcium.
Patient 3: Mr. G.C The discovery of his kidney disease incidentally revealed proteinuria in preventive medicine when he was 19 years old. Mr. B. R had no clinical manifestation. He showed no polydipsic polyuria syndrome, he never had any renal colic, did not show weight retardation and or bone deformity either.
His GFR under MDRD formula deteriorated to 58 ml/min (serum creatinine 135 mol/l). Calcium and phosphate profile was normal and there was no electrolyte disorder. His mixed proteinuria was predominantly tubular (Table 1). Genetic confirmation of the Dent's disease was made in 2010 showing a CIC-5 gene mutation, which was also objectified in his mother at the heterozygous state. There were nephrocalcinosis lesions associated with renal fibrosis. Bone densitometry showed z score at -2.1. Currently he is under IEC at optimal dose with good clinical and biological tolerance.
Patient 4: Mr.T.C He is the younger brother of Mr. GC. He had no particular history. The family genetic investigation showed that he was carrying the same mutation as his elder brother. He had no symptoms, at most proteinuria of 0.5 g/g with urinary calcium/creatinine level of 0.6. When he was 20, his kidney function seemed normal (creatinine concentration of 83 μmol/l). Calcium and phosphate levels were normal. Proteinuria was both glomerular and tubular; 0.45 ± 0.05 g/g on average. Micro albumin was about 130 mg/day.
Discussion
The Dent's disease was first described by Dent and Friedman in 1964 about two young English patients suffering from rickets associated with a gross tubular dysfunction, together with hypercalciuria, hyperphosphatiuria, proteinuria and amino aciduria [8]. In monitoring these patients, Wrong et al came across nephrolithiasis, and renal failure. [9] This disease is also known as Wrong-Dent disease in tribute to these pioneers, a rare disorder the prevalence of which is still unknown. About 250 families have been identified worldwide to date [10,11].
The clinical diagnosis (the term ‘clinical’ has to be deleted) lies on three joint criteria: (1) low molecular weight proteinuria with increased urinary excretion of β2-microglobulin and/or RBP at least 5 times above normal upper limit, (2) hypercalciuria greater than 4mg/kg/24 h in the 24-hour urines or over 0.25 mg/mg of creatinine concentration in a urine sample, (3) and at least one of the following elements: nephrocalcinosis, kidney lithiasis, hematuria, hypophosphatemia or kidney failure [12,13]. Three of our patients (1 to 3) fulfill these criteria. Patient 4 has two clinical criteria (‘clinical’ has to be deleted): PBM and hypercalciuria. Similar cases have been reported by authentic Dent’s disease confirmed by genetics [14,15]. In the presence of a mutation in the CLCN5 gene, only one of the above clinical (‘clinical’ has to be deleted) criteria is enough to make the diagnosis. Considering the first two clinical (‘clinical’ has to be deleted) criteria, only MBP is characteristic of the Dent’s disease. Indeed, although apparent very early, calcium excretion was observed in 90% of cases in Europe and the USA [16]. In Japan, in 86 cases unrelated to the disease, calcium excretion was observed only in 51% of them [17]. Proteinuria remains relatively constant in the range of 0.5 to 2 g per day for adults and 1 g per day in children [18,19,20] with less than 50% of microalbumnuria. Despite this disparity, clinical (‘clinical’ has to be deleted) criteria remain highly relevant for patients who meet them, genetic confirmation is obtained in 75% of cases.
The polyuria-polydipsic syndrome observed in patients # 1 and 2 and spontaneous hypokalemia in patient # 2 are common in the Dent's disease, however inconstant. Hypomagnesemia and hypocalcemia observed in patient # 2 are unusual for this disease. This might be linked to kidney failure.
The natural history of the Dent’s disease is characterized by early onset (patients # 1 and 2) at the very young age of PBM and / or hypercalciuria which remains without symptoms until possible occurrence of two complications: (1) rickets and/or weight retardation in children, and osteomalacia in adults, (2) at kidney level, urolithiasis or nephrocalcinosis often causing renal colic episodes. The role of nephrocalcinosis in the onset and progress of renal failure is uncertain. Nephrocalcinosis has no impact on the development and progression of renal failure [16]. 30 to 80% of affected males develop end stage renal disease (ESRD) between 30 and 50 years as evidenced by patient # 2 [21,22]. In some cases ESRD occurs around the sixth decade or later [23,24]. Deterioration of renal function may occur even in the absence of nephrocalcinosis [25]. Stones are made of calcium phosphate or a mixture of calcium phosphate and calcium oxalate [26]. It is important to emphasize inter and intra-familial heterogeneity of Dent disease. Indeed, at 20 years old, patients 1 and 2 had a chronic kidney disorder, whereas the disease is much less acute in the brothers (patients # 3 and 4).
Often small-sized in the case of DD. A standard deviation of - 0.58 and -2.10 in size was observed in a series in Case - 1 and Case -2 respectively [27].
The purpose of the treatment is to decrease urine calcium to prevent nephrocalcinosis or nephrolithiasis and slowing down progress of the kidney disease. The use of thiazide diuretics at higher doses at 0.4 mg/kg/day led to a 40% reduction of calciuria in patients [28]. However, side effects often limit their use as we have seen with patient # 2. Because of the component of glomerular proteinuria, angiotensin converting enzyme (ACE) inhibitors and angiotensin-II receptor antagonists (ARAII) are often used to slowdown the progress of renal disease. A Blanchard et al did found no benefit in using them [29]. Careful vitamin D (‘careful’ has to be deleted) supplementation is useful in patients with rickets or osteomalacia.
A slowing down in the progress of CKD is observed in CLCN5 knockout mice on high citrate diet [30] and so, it is often used in the treatment of Dent disease although no human clinical tests have yet demonstrated its effectiveness.
Conclusion
Advances in genetics have provided insights into the disease. However, the therapeutic means remain limited. Questions have not still been answered, namely the impact of ACE inhibitors and ARBs in slowing down the progress of CKD, 25-35% of cases observed without genetic mutation, and the exact reason why CIC-5 lesions are hosted by the loop of Henle and the intercalated cells.
References
- Jungers P, Joly D, Blanchard A, Courbebaisse M, Knebelmann B, et al. (2005)Lithiasesrénaleshéréditairesmonogéniques: recentsacquisdiagnostique et therapeutiques. Néphrologie et thérapeutique 4:231-255.
- Lloyd SE, Pearce SH, Fisher SE, Stein Meyer K, Schwappach B, et al. (1996) A common molecular basis for three inherited kidney stone disease. Nature 379:445-449.
- Wu F, Reed AA, Williams SE, Loh NY, Lippiat JD, et al. (2009) Mutational analysis of CLC-5, Cofilin and CLC-4 in patients with Dent’s disease. Nephron Physiol 112: 53-62.
- Shrimpton AE, HoopesRR, Knohl SJ, Hueber P, Reed AA, et al. (2009) OCRL1 mutations in Dent 2 patients suggest a mechanism for phenotypic variability. Nephron Physiol 112: 27-36.
- Jentsch TJ, Stein V,Weinreich F,Zdebik AA (2002) Molecular structure and physiological function of chloride channels. Physiol Rev 82: 503-568.
- Devuyst O, Christie PT, Courtoy PJ, Beauwens R, Thakker RV (1999) Intra-renal and subcellular distribution of human chloride channel, CLC-5, reveals a pathophysiological basis for Dent’s disease. Hum Mol Genet8: 247-257.
- Günther W,Lüchow A,Cluzeaud F,Vandewalle A,Jentsch TJ (1998) CIC-5, the chloride channel mutated in Dent’s disease, colocalizes with the proton pump in endocytotically active kidney cells. ProcNatlAcadSci USA 95: 8075-8080.
- Scheinman SJ (1998) X-linked hypercalciuric nephrolithiasis: clinical syndromes and chloride channel mutations. Kidney Int 53: 3-17.
- Wrong OM, Norden AG, Fest TG (1994) Dent’s disease: a familial proximal renal tubular syndrome with low-molecular-weight proteinuria, hypercalciuria, nephrocalcinosis, metabolic bone disease, progressive renal failure and a marked male predominance. Q J Med 87: 473-493.
- Wang SS,Devuyst O,Courtoy PJ,Wang XT,Wang H, et al. (2000) Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet 9: 2937-2945.
- Cebotaru V,Kaul S,Devuyst O,Cai H,Racusen L, et al. (2005) High citrate diet delays progression of renal insufficiency in the CIC-5 knockout mouse model of Dent’s disease. Kidney Int, 68: 642-652.
- Dent CE, Friedman M (1964)Hypercalcuric rickets associated with renal tubular domage. Arch Dis Child 39: 240-9.
- Hryciw DH, Wang Y, Devuyst O, Pollok CA, Poronnik P, et al. (2003) Cofilin interacts with CIC-5 and regulates albumin uptake in proximal tubule cell lines. J BiolChem278: 40169-40176.
- Kumar R (1998) CLC-5 chloride channels and renal disease. Kidney Int 53: 228-9.
- Jouret F, Courtois PJ, Devuyst O (2006)Lithiaserénale et tubulopathiecomplexe: le paradigme de la maladie de Dent. Actual Nephrol 36: 105-17.
- Matteis MAD, Luini A (2011) Mendelian disorders of membrane trafficking. N ENG J MED 365: 927-38.
- Scheel O, Zdebik AA, Lourdel S, Jentsch TJ (2005) Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436: 424-427.
- Jentsch TJ (2007) Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol 578: 633-640.
- Zhang X, Hartz PA, Philip E, Racusen LC, Majerus PW (1998) Cell lines from kidney proximal tubules of a patient with Lowe syndrome lack OCRL inositol polyphosphate 5-phosphatase and accumulate phosphatidylinositol 4,5-bisphosphate. J BiolChem 273: 1574-1582.
- Devuyst O, Thakker RV (2010) Dent’s disease. Orphanet J Rare disease 5: 28.
- Sekine T,Komoda F,Miura K,Takita J,Shimadzu M (2013) Japanese Dent disease has a wider clinical spectrum than Dent disease in Europe/USA: genetic and clinical studies of 86 unrelated patients with low-molecular-weight proteinuria. Nephrol Dial Transplant 29: 376-384.
- Böckenhauer D, Bökenkamp A, Nuutinen M,Michael Ludwig (2012) Novel OCRL mutations in patients with Dent-2 disease. J Pediatr Genet 1: 15-23.
- Frymoyer PA, Scheinman SJ, Dunham PB, Jones DB, Hueber P, et al. (1991) X-linked recessive nephrolithiasis with renal faiilure. N Eng J Med 325: 681-686.
- Scheinman SJ, Cox JPD, Lloyd SE, Pearce SHS, Salenger PV, et al. (2000) Isolated hypercalciuria with mutation in CLCN5: Relevance to idiopathic hypercalciuria. Kidney Int 57: 232-239.
- Frishberg Y, Dinour D, Belostotsky R, Becker-Cohen R, Rinat C, et al. (2009)Dent’s disease manifesting as focal glomerulosclerosis: Is it the tip of iceberg? PediatrNephrol 24: 2369-2373.
- Claverie-Martin F, Ramos-Trujillo E, Garcia-Nieto V (2011) Dent’s disease: clinical features and molecular basis. PediatrNephrol26: 693-704.
- Igarashi T,Hayakawa H,Shiraga H,Kawato H,Yan K, et al (1995). Hypercalciuria and nephrocalcinosis in patients with idiopathic low-molecular-weight proteinuria in Japan: is the disease identical to Dent’s disease in United Kingdom? Nephron 69: 242-247.
- Lloyd SE, Pearce SHS, Guenther W, Kawaguchi H, Igarashi T, et al. (1997) Idiopathic low molecular weight proteinuria associated with hypercalciuricnephrocalcinosis in Japanese children is due to mutations of the renal chloride channel (CLCN5). J Clin Invest. 99: 967-74.
- Bökenkamp A, Böckenhauer D, Cheong HI, Hoppe B, Tasic V, et al. (2009) Dent-2 disease: a mild variant of Lowe syndrome. J Pediatr155: 94-9.
- Raja KA, Schurman S, M’Mello R G, Blowey D, Goodyer P, et al. (2002) Responsiveness of hypercalciuria to thiazide in Dent’s disease. J Am SocNephrol 13: 2938-44.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences