Decreased Glycaemia with Renal Failure in Diabetes Betides in Relation to the Change in Renal Glutamate Metabolism
Susumu Ogawa, Manami Shimizu, Kazuhiro Nako, Masashi Okamura and Sadayoshi Ito
DOI10.21767/2472-5056.100055
Susumu Ogawa1,2*, Manami Shimizu1, Kazuhiro Nako1, Masashi Okamura1 and Sadayoshi Ito1
1Division of Nephrology, Endocrinology and Vascular Medicine, Tohoku University Hospital, 1-1 Seiryo-machi, Aoba-ku, Sendai 980-8574, Japan
2Department of Clinical Medicine, Division of Research in Student Support, Institute for Excellence in Higher Education, Section of Clinical Medicine, Tohoku University, Sendai, Japan
- *Corresponding Author:
- Susumu Ogawa
Division of Nephrology, Endocrinology and Vascular Medicine, Tohoku University Hospital, Japan
E-mail: ogawa-s@hosp.tohoku.ac.jp
Received date: February 06, 2018; Accepted date: February 20, 2018; Published date: February 27, 2018
Citation: Ogawa S, Shimizu M, Nako K, Okamura M, Ito S (2018) Decreased Glycaemia with Renal Failure in Diabetes Betides in Relation to the Change in Renal Glutamate Metabolism. J Clin Exp Nephrol Vol 3:4. DOI: 10.21767/2472-5056.100055
Copyright: © 2018 Ogawa S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
A reduction in renal gluconeogenesis appears to be one cause for the drop in blood glucose concentration (BGC) accompanying decreased renal function in diabetes. However, it remains unclear as to how this drop in BGC is related to the changes in reabsorption of amino acids (AAs) that accompany decreased renal function. We therefore investigated the relationship between the drop in BGC accompanying decreased renal function in diabetes patients and changes in the reabsorption rates of AAs.
Subjects and methods: Using 100 diabetes patients and 100 non-diabetics as subjects, we measured their blood AAs concentration and urinary AAs excretion, and calculated the AAs’ reabsorption rate. We then examined the relationship between the latter and the estimated glomerular filtration rate (eGFR) and HbA1c.
Results: In diabetics, blood glutamate concentration and reabsorption rate of glutamate were reduced. The blood glutamine concentration was increased; however, the reabsorption of glutamine was unchanged. The reabsorption rates of some AAs, including glutamate, showed a positive correlation with eGFR. However, a reduced reabsorption rate of glutamate was the only independent risk factor for reduced eGFR. Moreover, only the reabsorption rate of glutamate correlated positively with HbA1c.
Conclusions: In diabetes, glutamate reabsorption shows a decline that parallels decreased renal function: this reduction is related to a drop in BGC. The reduction in the reabsorption of glutamate appears to influence renal gluconeogenesis by reducing the gluconeogenesis-adjusting factor (malate-aspartate shuttle), not by reducing gluconeogenic substrates. Further studies are therefore needed to examine the role glutamate plays in renal gluconeogenesis.
Keywords
Diabetic nephropathy; Reduced glycaemia; Glutamate; Glutamine; Gluconeogenesis
Introduction
In diabetes, a phenomenon is seen in which blood glucose concentration (BGC) drops in parallel with the progression of nephropathy, often making it difficult for physicians to prevent hypoglycaemia [1]. Diabetic patients with decreased renal function may appear to manifest low HbA1c values due to renal anaemia and subsequent treatments using erythropoietin preparations. In spite of being corrected, however, BGC continues to drop in parallel with loss of renal function. Traditionally, slower insulin metabolism and excretion by the kidneys was thought to be one cause for this drop in BGC [1-3]. However, considering few diabetes patients with low renal function manifest hyperinsulinemia, it is difficult to explain how renal failure alone can cause hypoglycaemia due solely to low insulin clearance.
Between 20% and 50% of endogenous glucose release is provided by renal glucose release [4]. Renal glucose release is performed only with gluconeogenesis in the renal proximal tubules (RPTs) [5] and the substrates of renal gluconeogenesis are lactate, glycerol, and glutamine in the blood [6]. Lactate is changed into pyruvate; the pyruvate enters the mitochondria and is used in gluconeogenesis. Glutamine, on the other hand, becomes α-ketoglutarate by way of glutamate and is used for gluconeogenesis. Insulin suppresses the kidneys’ glucose release, but does not control the uptake of glucose, lactate, glycerol, or glutamine. There is a possibility that BGC drops in renal failure because of this decline in renal gluconeogenesis. There are several reports describing this phenomenon [7].
Several types of amino acids in the blood, such as glutamine, are taken into the RPTs from the basolateral side. The majority of amino acids in the blood, however, are completely filtered by the glomeruli and amino acids are then almost completely reabsorbed into the RPTs from the apical side [8]. In other words, many glucogenic amino acids, including glutamate, are absorbed inside the RPTs by reabsorption from the apical side but not from the basolateral side.
However, there are few studies on the use of these glucogenic amino acids once reabsorbed into the RPTs for gluconeogenesis purposes. Therefore, we measured the blood concentration of amino acids in diabetics and non-diabetics, as well as their urinary amino acids excretion, evaluated the renal reabsorption rate of amino acids, and examined the relationship between these and decreased renal function.
Materials and Methods
This is a cross-sectional study of 200 subjects: 100 nondiabetic, normal renal function, obese our university students who had undergone health check-ups (control group: CG), and 100 diabetic patients who were undergoing outpatient treatment at our university Hospital (diabetic group: the DG). All the subjects provided their informed consent to participate in this study.
In DG, individuals who were being treated with erythropoietin preparations, presented marked renal anaemia, and carried the risk of having their HbA1c values modified, such as those with elevated reticulocyte levels, were excluded from this study. We also excluded the following patients from this study: those whose BGCs were rapidly changing because of changes in their treatment drugs or other reasons; those who were at risk of having their glycoalbumin (GA) values affected due to reasons other than BGC, such as hypoalbuminemia; and those who showed large differences between their GA values estimated from HbAlc and actual measured GA values (e.g., abnormal HbA1c). We measured the subjects’ blood amino acids concentrations and urinary amino acids excretion and calculated each amino acid’s reabsorption rate (reabsorption) using the following formula: [1–urinary amino acid excretion (μmol/g creatinine) × serum creatinine (mg/dL)/blood amino acid level (μmol/L) × 100] × 100 (%) [9].
To measure amino acids, we used a previously reported method (Supplementary document) [10]. We then examined the correlation between the estimated glomerular filtration rate (eGFR) and urinary albumin excretion (urinary albumin-tocreatinine ratio: ACR), which are important clinical indicators of renal function in diabetic nephropathy, as well as the correlation between HbA1c (NGSP value) and GA, which are indicators of glycaemic control, and the reabsorptions. We studied the correlation between each reabsorptions and renal function (eGFR and ACR), and examined the relationship between the indicators of glycaemic control (HbA1c and GA) and each Reabsorptions. We also examined the reabsorptions that were related to both decreased renal function and a drop in BGC.
This study complies with the Helsinki Declaration and was conducted with the approval of the Medical Ethics Committee of Tohoku University. All participants provided their full informed consent.
Statistical analysis
Numerical figures that were normally distributed are shown as mean ± SD and those that did not, as median (range). Single correlations were studied using Spearman’s test. If there were multiple factors that correlated individually, we performed a multiple regression analysis and searched for independent factors. p<0.05 was regarded as statistically significant.
Results
The study subjects’ basic characteristics data were shown in the Table 1. The subjects in DG had already been given numerous treatment drugs (Supplementary Table 1). The subjects in CG had not received treatment at all. In the DG, the eGFR and HbA1c correlated positively (r=0.39, p<0.01), but ACR and HbA1c did not (r=-0.14, p=0.18).
| Control group | Diabetic group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | ||||||
| Age | Years | 22.3 | ± | 3.4 | 62.4 | ± | 15.1 | <0.01 | ||
| Gender | M/F | 92 / 8 | 41/59 | <0.01 | ||||||
| Body mass index | kg/m2 | 32.6 | ± | 2.1 | 26.1 | ± | 5.7 | <0.01 | ||
| Systolic blood pressure | mmHg | 133 | ± | 11.5 | 128.8 | ± | 17.1 | 0.04 | ||
| Diastolic blood pressure | mmHg | 74.5 | ± | 11.3 | 70.2 | ± | 12 | 0.01 | ||
| Urinary pH | 5.9 | ± | 0.5 | 5.9 | ± | 0.8 | 0.74 | |||
| eGFR | mL/min/1.73 m2 | 97.8 | ± | 15.3 | 66.3 | ± | 31.2 | <0.01 | ||
| Aspartate transaminase | IU/L | 29.4 | ± | 17.3 | 23.7 | ± | 10.9 | 0.01 | ||
| Alanine transaminase | IU/L | 55.5 | ± | 44.9 | 22.5 | ± | 15.1 | <0.01 | ||
| Plasma glucose | mmol/L | 4.9 | ± | 0.8 | 7.8 | ± | 2.5 | <0.01 | ||
| HbA1c (IFCC) | mmol/mol | - | - | 53.5 | ± | 11.8 | - | |||
| Serum total cholesterol | mmol/L | 4.9 | ± | 0.9 | 4.6 | ± | 0.8 | 0.04 | ||
| Serum triglyceride | mmol/L | 1.6 | ± | 0.9 | 1.4 | ± | 0.9 | 0.05 | ||
| HDL-C | mmol/L | - | - | 1.5 | ± | 0.5 | - | |||
| Blood urea nitrogen | mmol/L | 4.5 | ± | 1 | 7.2 | ± | 5.5 | <0.01 | ||
| Serum uric acid | µmol/L | 432.1 | ± | 94.9 | 323.7 | ± | 93.4 | <0.01 | ||
| ACR | mg/g Cr | 24.4 (3.3–6710) | - | |||||||
| 8-OHdG | ng/g Cr | 3.7 | ± | 1.9 | 8.9 | ± | 3.5 | <0.01 | ||
SD: Standard deviation; eGFR: Estimated glomerular filtration rate; HbA1c: Hemoglobin A1c; IFCC: International Federation of Clinical Chemistry; HDL-C: Serum high density lipoprotein cholesterol; ACR: albumin-to-creatinine ratio (urinary albumin excretion); 8- OHdG: Urinary 8-hydroxy-2'-deoxyguanosine excretion.
Table 1: Clinical characteristics seen in the non-diabetic group and the diabetic group.
Although eGFR correlated positively with GA (r=0.32, p<0.01), the degree of correlation was weaker than that between eGFR and HbA1c. GA did also not correlate with ACR (r=-0.09, p=0.39); conversely, HbA1c and GA showed a strong positive correlation (r=0.94, p<0.01). In addition, although HbA1c was measured in all subjects, GA measurement values were missing in several subjects. Therefore, we decided to use HbA1c instead of GA as an indicator of glycaemic control.
Table 2 shows the results for blood amino acids concentration and urinary amino acids excretion. The reabsorptions are presented in Table 3. The reabsorptions of numerous amino acids exceeded 90% (most were over 98%); however, some showed relatively low reabsorptions, such as cysteine, cystathionine, m-methanol amine, 1-methyl histidine, and 3- methyl histidine.
| Blood amino acids (μmol/dL) | Urinary amino acids (μmol/g Cr) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control group | Diabetes group | Control group | Diabetes group | |||||||||||
| Mean | SD | Mean | SD | p1 | Mean | SD | Mean | SD | p2 | |||||
| Taurine | 42 | ± | 8.2 | 82.2 | ± | 55.9 | <0.01 | 556.3 | ± | 717.5 | 987.7 | ± | 1207.4 | <0.01 |
| PPEA | 36.5 | ± | 16.7 | 42.2 | ± | 16.6 | 0.02 | |||||||
| Aspartic acid | 14.9 | ± | 5.8 | 5.3 | ± | 1.3 | <0.01 | 12.8 | ± | 4.8 | 26 | ± | 20 | <0.01 |
| HP | 13.5 | ± | 8.1 | 12.6 | ± | 7.8 | 0.39 | 13.5 | ± | 8.9 | 55.6 | ± | 93.8 | 0.05 |
| Threonine | 128.5 | ± | 27.9 | 125.3 | ± | 31.2 | <0.01 | 110.2 | ± | 104.8 | 248.5 | ± | 364 | <0.01 |
| Serine | 114.3 | ± | 20.7 | 118.4 | ± | 25.3 | <0.01 | 279.6 | ± | 147.6 | 491.5 | ± | 382.2 | <0.01 |
| Asparagine | 38.8 | ± | 9.4 | 55.8 | ± | 12.2 | <0.01 | 78.9 | ± | 51.8 | 176.2 | ± | 176.4 | <0.01 |
| Glutamate | 259.9 | ± | 89.8 | 62.6 | ± | 20.3 | <0.01 | 19.3 | ± | 8.6 | 34.3 | ± | 27.8 | <0.01 |
| Glutamine | 286.5 | ± | 95 | 530.6 | ± | 78.3 | <0.01 | 302.5 | ± | 138.5 | 424.7 | ± | 352.2 | <0.01 |
| AAAA | - | 29.4 | ± | 14.7 | 41.8 | ± | 29 | <0.01 | ||||||
| Proline | 203.1 | ± | 56.6 | 174.8 | ± | 51.4 | <0.01 | 26.8 | ± | 11.1 | 80.3 | ± | 104.7 | <0.01 |
| Glycine | 200.5 | ± | 32.6 | 237 | ± | 79.6 | <0.01 | 657.1 | ± | 442.9 | 1335.1 | ± | 1150.3 | <0.01 |
| Alanine | 457.8 | ± | 84.5 | 431.5 | ± | 95.9 | <0.01 | 198.7 | ± | 114.2 | 480.9 | ± | 418.6 | <0.01 |
| Citrulline | 21.9 | ± | 5.4 | 36.2 | ± | 28.3 | <0.01 | 11.4 | ± | 3.6 | 59.1 | ± | 87.9 | <0.01 |
| AABA | 20.8 | ± | 7.1 | 19.3 | ± | 7.2 | <0.01 | 17.4 | ± | 8.9 | 29.5 | ± | 12.1 | <0.01 |
| Valine | 266.8 | ± | 43.5 | 226.7 | ± | 46.9 | 0.02 | 26.8 | ± | 6.6 | 57.6 | ± | 60.3 | <0.01 |
| Cysteine | 1.3 | ± | 0.5 | 16.9 | ± | 15.6 | <0.01 | 49.6 | ± | 24.6 | 109.1 | ± | 131.4 | <0.01 |
| Methionine | 21.4 | ± | 7.1 | 23.8 | ± | 6 | <0.01 | 6.6 | ± | 3.3 | 21.7 | ± | 19 | 0.05 |
| Cystathionine | - | 1.5 | ± | 0.6 | <0.01 | 13.7 | ± | 8.2 | 21 | ± | 12.2 | <0.01 | ||
| Isoleucine | 83.6 | ± | 21.6 | 67.4 | ± | 19 | <0.01 | 9.5 | ± | 2.6 | 36.2 | ± | 33.5 | <0.01 |
| Leucine | 147.6 | ± | 32.3 | 117.5 | ± | 31 | <0.01 | 24.4 | ± | 7.3 | 45 | ± | 42.5 | <0.01 |
| Tyrosine | 73.5 | ± | 17.1 | 58.5 | ± | 17.8 | <0.01 | 132.5 | ± | 147.3 | 91.5 | ± | 77.8 | <0.01 |
| Beta alanine | 11.2 | ± | 4 | 9.3 | ± | 1.7 | <0.01 | 26.5 | ± | 19.6 | 56.3 | ± | 35.5 | <0.01 |
| Phenylalanine | 65.1 | ± | 11.7 | 59.9 | ± | 9.5 | <0.01 | 37.8 | ± | 13.2 | 62.8 | ± | 41 | <0.01 |
| BAIBA | 2.5 | ± | 1 | 4.3 | ± | 4.1 | <0.01 | 338.8 | ± | 382.4 | 500.1 | ± | 578.6 | <0.01 |
| Methanolamine | 11.3 | ± | 3 | 7.6 | ± | 1.9 | <0.01 | 302.2 | ± | 79 | 272.9 | ± | 117.6 | <0.01 |
| Hydroxylysine | 7.4 | ± | 5.2 | 24.9 | ± | 31.9 | <0.01 | |||||||
| Ornithine | 84 | ± | 24 | 79.8 | ± | 20.8 | <0.01 | 12.4 | ± | 5.4 | 43.9 | ± | 52.7 | <0.01 |
| 1MH | 7.5 | ± | 6.4 | 9.3 | ± | 10.1 | <0.01 | 506.3 | ± | 488 | 492.3 | ± | 521.7 | <0.01 |
| Histidine | 77.5 | ± | 11.4 | 77.6 | ± | 10.7 | <0.01 | 717.5 | ± | 338.9 | 715.4 | ± | 489.6 | <0.01 |
| Lysine | 197.3 | ± | 41.8 | 194 | ± | 36.7 | <0.01 | 174 | ± | 164 | 288.5 | ± | 390 | <0.01 |
| 3MH | 5.6 | ± | 2.7 | 7.1 | ± | 5.5 | 0.02 | 258.2 | ± | 62.8 | 304.8 | ± | 60.8 | <0.01 |
| Tryptophan | 56.8 | ± | 9.9 | 41 | ± | 10.6 | <0.01 | 53.1 | ± | 19.9 | 68.8 | ± | 47.6 | <0.01 |
| Anserine | 92.7 | ± | 70.2 | 265.3 | ± | 299 | 0.95 | |||||||
| Carnosine | 16 | ± | 14.8 | 46 | ± | 35.3 | 0.47 | |||||||
| Arginine | 61.3 | ± | 20.7 | 67 | ± | 21.1 | <0.01 | 19.7 | ± | 6.9 | 49.2 | ± | 42.3 | <0.01 |
| Fisher ratio | 3.6 | ± | 0.5 | 3.5 | ± | 0.7 | 0.17 | |||||||
Table 2: A comparison of blood amino acids levels (BAA) and urinary amino acids excretion (UAA) between the control group and the diabetic group.
| Control group | Diabetic group | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |||
| Taurine | 89.3 | ± | 11.61 | 87.74 | ± | 14.7 | 0.41 |
| Aspartic acid | 99.14 | ± | 0.6 | 96.18 | ± | 7.88 | <0.01 |
| Hydroxy proline | 99 | ± | 0.77 | 95.6 | ± | 7.33 | <0.01 |
| Threonine | 99.33 | ± | 0.49 | 97.68 | ± | 5.63 | <0.05 |
| Serine | 97.99 | ± | 0.9 | 95.66 | ± | 6.31 | <0.01 |
| Asparagine | 98.29 | ± | 1.03 | 96.72 | ± | 5.47 | 0.01 |
| Glutamate | 99.93 | ± | 0.04 | 99.42 | ± | 0.78 | <0.01 |
| Glutamine | 99.03 | ± | 0.61 | 99.13 | ± | 1.61 | 0.58 |
| Sarcosine | - | - | - | - | - | ||
| Alpha-aminoadipic acid | - | - | - | - | - | ||
| Proline | 99.89 | ± | 0.06 | 99.32 | ± | 2.18 | 0.01 |
| Glycine | 97.36 | ± | 1.49 | 94.63 | ± | 6.35 | <0.01 |
| Alanine | 99.64 | ± | 0.2 | 98.8 | ± | 1.9 | <0.01 |
| Citrulline | 99.57 | ± | 0.13 | 98.92 | ± | 2.04 | <0.01 |
| Alpha-aminobutyric acid | 99.28 | ± | 0.41 | 98.05 | ± | 3.2 | <0.01 |
| Valine | 99.92 | ± | 0.02 | 99.69 | ± | 0.78 | <0.01 |
| Cysteine | 70.53 | ± | 11.6 | 88.09 | ± | 16.97 | <0.01 |
| Methionine | 99.73 | ± | 0.15 | 99.46 | ± | 0.9 | <0.01 |
| Cystathionine | - | - | 37.99 | ± | 26.18 | <0.01 | |
| Isoleucine | 99.9 | ± | 0.03 | 99.78 | ± | 0.51 | 0.01 |
| Leucine | 99.86 | ± | 0.04 | 99.59 | ± | 1.01 | 0.01 |
| Tyrosine | 98.4 | ± | 2.09 | 98.37 | ± | 2.94 | 0.93 |
| Beta alanine | 97.97 | ± | 1.36 | 93.33 | ± | 8.18 | <0.01 |
| Phenylalanine | 99.52 | ± | 0.15 | 98.88 | ± | 1.75 | <0.01 |
| Beta-aminoisobutyric acid | -63.76 | ± | 88.95 | -18.48 | ± | 74.01 | <0.01 |
| Homocysteine | - | - | - | - | - | ||
| Gamma-aminobutyric acid | - | - | - | - | - | ||
| Methanolamine | 76.77 | ± | 7.23 | 69.89 | ± | 9.19 | <0.01 |
| Hydroxylysine | - | - | - | - | - | ||
| Ornithine | 99.87 | ± | 0.08 | 99.44 | ± | 1.42 | <0.01 |
| 1-methyl histidine | 37.16 | ± | 22.48 | 41.67 | ± | 24.16 | 0.19 |
| Histidine | 92.41 | ± | 3.32 | 91.83 | ± | 7.17 | 0.47 |
| Lysine | 99.25 | ± | 0.72 | ± | 0.02 | ||
| 3-methyl histidine | 60.53 | ± | 7.31 | ± | <0.01 | ||
| Tryptophan | 99.22 | ± | 0.3 | ± | <0.01 | ||
| Arginine | 99.7 | ± | 0.19 | ± | <0.01 | ||
Table 3: Comparisons of the RPTs’ AA reabsorption rate between the CG and the DG.
Table 4 shows the correlation between various amino acids’ Reabsorptions and HbA1c, and eGFR. The eGFR correlated positively with the reabsorptions of proline, glutamate, alanine, serine, phenylalanine, tryptophan, asparagine, valine, arginine, and glycine, but correlated negatively with the reabsorptions of cysteine and isoleucine (Supplementary Table 2).
Therefore, we designated eGFR as the dependent variable and used the reabsorption of proline, glutamate, alanine, serine, phenylalanine, tryptophan, asparagine, valine, arginine, and glycine as the independent variables in a multiple regression analysis. As a result, only the reabsorption of glutamate was identified as an independent factor (β=12.36, p=0.03, 95% CI=1.16–23.57) (Supplementary Table 3).
In other words, it was confirmed that, along with a reduction in eGFR, the reabsorption of glutamate had continued to drop. HbA1c correlated with the reabsorptions of several amino acids (Table 4).
| eGFR | HbA1c | eGFR | HbA1c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Reabsorption rate (%) | r | p | r | p | |||||
| Taurine | 0.46 | <0.01 | 0.13 | 0.19 | Cystathionine | 0.89 | 0.02 | -0.39 | 0.44 |
| Aspartate | 0.17 | 0.1 | -0.03 | 0.77 | Isoleucine | -0.27 | 0.01 | -0.22 | 0.03 |
| Hydroxyproline | 0.38 | <0.01 | -0.06 | 0.55 | Leucine | 0.02 | 0.84 | -0.14 | 0.17 |
| Threonine | 0.19 | 0.06 | -0.08 | 0.41 | Tyrosine | 0.17 | 0.1 | -0.11 | 0.29 |
| Serine | 0.36 | <0.01 | -0.05 | 0.64 | Beta alanine | 0.71 | <0.01 | 0.23 | 0.02 |
| Asparagine | 0.28 | <0.01 | -0.07 | 0.48 | Phenylalanine | 0.36 | <0.01 | -0.05 | 0.64 |
| Glutamate | 0.41 | <0.01 | 0.21 | 0.03 | baiba | -0.17 | 0.13 | -0.12 | 0.31 |
| Glutamine | 0.14 | 0.18 | -0.03 | 0.76 | MEA | 0.31 | 0 | 0.05 | 0.64 |
| Proline | 0.57 | <0.01 | 0.06 | 0.56 | Ornithine | 0.07 | 0.51 | -0.28 | <0.01 |
| Glycine | 0.2 | 0.05 | -0.17 | 0.1 | 1MH | -0.31 | <0.01 | -0.21 | 0.04 |
| Alanine | 0.4 | <0.01 | -0.09 | 0.35 | Histidine | 0.06 | 0.52 | -0.15 | 0.15 |
| Citrulline | 0.11 | 0.29 | -0.09 | 0.38 | Lysine | 0.02 | 0.87 | -0.22 | 0.03 |
| aaba | 0.51 | <0.01 | 0.1 | 0.3 | 3MH | -0.19 | 0.06 | -0.2 | 0.05 |
| Valine | 0.27 | 0.01 | -0.08 | 0.46 | Tryptophan | 0.32 | <0.01 | -0.03 | 0.8 |
| Cystine | -0.32 | <0.01 | -0.43 | <0.01 | Arginine | 0.23 | 0.02 | -0.18 | 0.08 |
| Methionine | -0.11 | 0.27 | -0.27 | 0.01 | |||||
aaba: Alpha-amino butyric acid; baiba:Beta-amino iso butyric acid; mea: m-ethanol amine; 1mh:1-methyl histidine; 3mh: 3-methyl histidine; the item that the under line is drawn is glucogenic amino acids
Table 4: Correlation between the subjects’ eGFR/HbA1c and amino acid reabsorption rates.
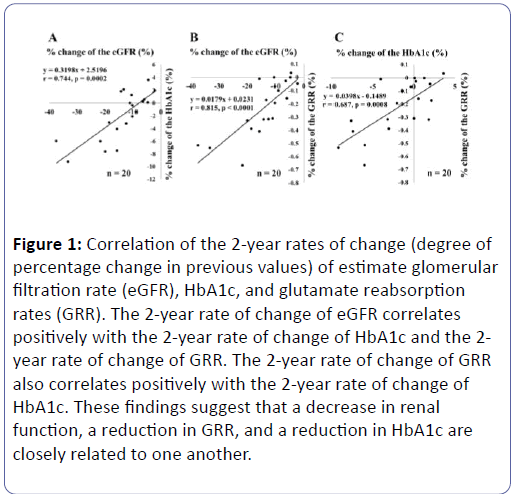
However, these were mostly negative correlations, and only glutamate showed a positive correlation (r=0.21, p=0.03) (Table 5). These showed that the reabsorption of glutamate declined in line with falling renal function (eGFR) and that HbAlc declined in line with this drop in the reabsorption of glutamate. These results suggest the possibility that a decline in eGFR reduces glutamate reabsorption, which in turn lowers the level of HbA1c. Therefore, we conducted a retrospective study using, as targets, subjects with eGFR <45 mL/min/1.73 m2 (those who were highly likely to see their renal function decline rapidly; n=30) and those whose data could be gathered two years later. We investigated the relationship among the subjects’ rates of change in eGFR (%) during a two-year period [the percentage of their previous values that had changed], the rates of change of HbA1c (%), and the rates of change of their glutamate reabsorptions (%) (Figure 1). The following subjects were excluded from the study: those whose treatment program had changed; those who had begun using erythropoietin preparations; those who were judged to be showing changes in BGCs due to changes in their lifestyles; and those whose anaemia had aggravated. As a result, a total of twenty individuals qualified for our final analysis. A positive correlation was seen between the rates of change in eGFR and in HbA1c (Figure 1A), between the rates of change in eGFR and in the glutamate reabsorptions (Figure 1B), and glutamate reabsorptions and rates of change of HbA1c (Figure 1C). These relationships remained unaffected even if GA was used in place of HbA1c. However, HbA1c showed stronger correlations in each case.
| Reabsorption rate (%) | HbA1c | |
|---|---|---|
| r | p | |
| Cysteine | −0.4304 | <0.0001 |
| Ornithine | −0.2833 | 0.0043 |
| Methionine | −0.2661 | 0.0074 |
| Beta alanine | −0.2277 | 0.0227 |
| Lysine | −0.2233 | 0.0255 |
| Isoleucine | −0.2227 | 0.0259 |
| Glutamate | 0.2128 | 0.0336 |
| 1-methylhistidine | −0.2085 | 0.0437 |
Table 5: A single correlation between HbA1c and various amino acids’ reabsorption rates.
Figure 1: Correlation of the 2-year rates of change (degree of percentage change in previous values) of estimate glomerular filtration rate (eGFR), HbA1c, and glutamate reabsorption rates (GRR). The 2-year rate of change of eGFR correlates positively with the 2-year rate of change of HbA1c and the 2- year rate of change of GRR. The 2-year rate of change of GRR also correlates positively with the 2-year rate of change of HbA1c. These findings suggest that a decrease in renal function, a reduction in GRR, and a reduction in HbA1c are closely related to one another.
Conclusion
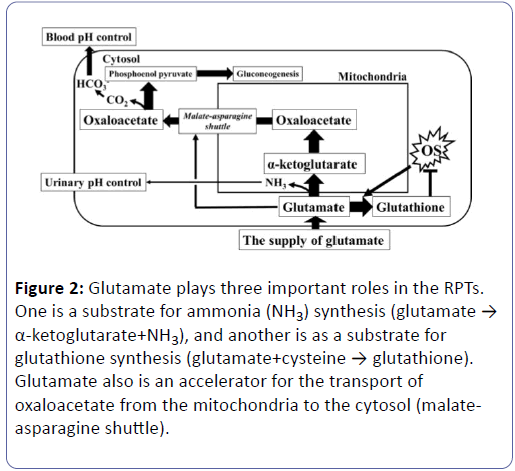
Glutamate plays some important roles in the RPTs in terms of various metabolisms (Figure 2). One role is to serve as a substrate for ammonia (NH3) synthesis (glutamate → α- ketoglutarate+NH3), and another is as a substrate for glutathione synthesis (glutamate+cysteine → glutathione). There also is a role for the transport of oxaloacetate from the mitochondria to the cytosol (malate-asparagine shuttle).
Figure 2: Glutamate plays three important roles in the RPTs. One is a substrate for ammonia (NH3) synthesis (glutamate → α-ketoglutarate+NH3), and another is as a substrate for glutathione synthesis (glutamate+cysteine → glutathione). Glutamate also is an accelerator for the transport of oxaloacetate from the mitochondria to the cytosol (malateasparagine shuttle).
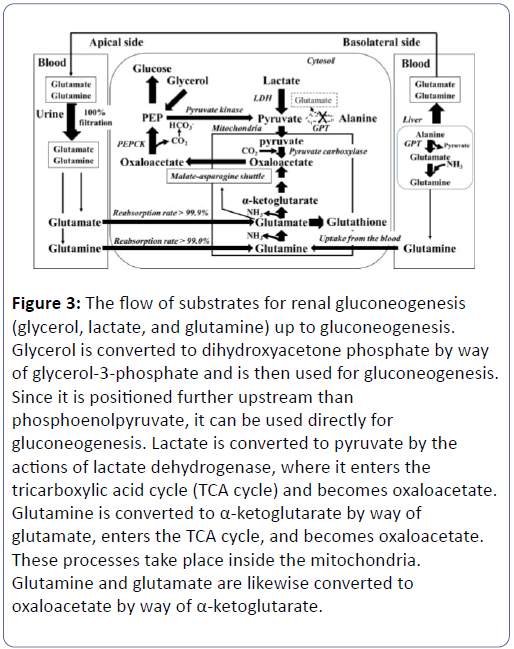
Therefore, a shortage of glutamate in the RPTs impedes a variety of metabolic activities. A reduction in NH3 synthesis lowers urinary pH [11-13] while an increase in oxidative stress causes an increase in glutathione synthesis leading to the consumption of glutamate. A reduction in the malate-asparagine shuttle rotation, moreover, induces a reduction in gluconeogenesis and a decline in HCO3 - synthesis, leading to the risk of causing decreased glycaemia and metabolic acidosis (Figure 2) [14-19]. During times of increased oxidative stress, moreover, glutathione synthesis increases, and glutamate ends up being consumed for this synthesis. As a result, the supply of glutamate to reactions other than glutathione synthesis decreases. Increasing the supply of glutamate to meet increased demand for glutamate is a complicated process (Figure 2) [20,21]. Blood glutamate is filtered 100% at the renal glomeruli, and close to 100% (>99.9%) is reabsorbed in the RPTs (Figure 3). Since the reabsorption is close to 100%, it is not possible to increase this rate any further [22,23]. If the blood glutamate concentration rose, the amount of reabsorption will increase accordingly. With diabetes, however, urinary glutamate excretion increases, the blood glutamate concentration drops, and the reabsorption also decline (Table 6). This shows that the supply of glutamate into RPT decreases in the diabetes. In addition, eGFR and the reabsorption of glutamate show a strong positive correlation (Table 4) (Supplementary Tables 2 and 3). This shows that DG included a large number of subjects with reduced renal function, and that, along with this decreased renal function, the reabsorption of glutamate dropped. The fact that the reabsorption of glutamate had dropped because of reduced renal function and the blood concentration has dropped may mean that, even if the decline in reabsorption is minor (0.51%), the amount of glutamate supplied to the RPTs is believed to drop significantly (Table 6). Vice versa, a decrease of glutamate reabsorption might possibly decrease the eGFR [24-26]. In contrast, since the blood glutamine concentration rises but its reabsorption remains unchanged, the amount of glutamine supply increased (Table 6). Glutamine also has reabsorption of close to 100 % (>99.0%), so there is almost no range of increases in the reabsorption amount significantly affecting its blood concentration or usage in the kidneys. The shortage of glutamate in the RPTs is compensated for by increasing the taking of glutamine from the blood (Figure 3) [27].
Figure 3: The flow of substrates for renal gluconeogenesis (glycerol, lactate, and glutamine) up to gluconeogenesis. Glycerol is converted to dihydroxyacetone phosphate by way of glycerol-3-phosphate and is then used for gluconeogenesis. Since it is positioned further upstream than phosphoenolpyruvate, it can be used directly for gluconeogenesis. Lactate is converted to pyruvate by the actions of lactate dehydrogenase, where it enters the tricarboxylic acid cycle (TCA cycle) and becomes oxaloacetate. Glutamine is converted to α-ketoglutarate by way of glutamate, enters the TCA cycle, and becomes oxaloacetate. These processes take place inside the mitochondria. Glutamine and glutamate are likewise converted to oxaloacetate by way of α-ketoglutarate.
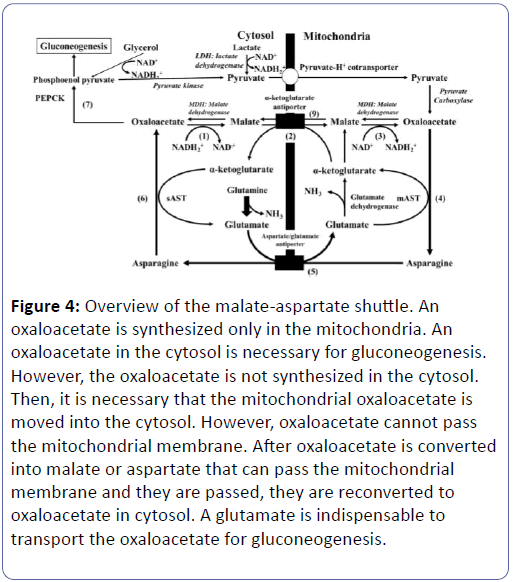
This shows that, in a diabetic or renal dysfunctional state, the demand for glutamate increases in the RPTs, and reabsorption of glutamate only cannot sufficiently meet this demand. Therefore, rather than increasing glutamine’s reabsorptions, the blood glutamine concentration is elevated to cope with the situation (Supplementary Figure 1). As a result, not only glutamine’s reabsorption amount slightly increases, but glutamine taken from the blood also appears to increase like supplementing the lack [28]. Glutamine becomes glutamate+NH3, so it can replenish glutamate. In a diabetic state, intra-RPT OS has largely increased, and glutathione is deemed to have significantly accelerated. As a result, consumption of glutamate has most likely increased, raising the demand for glutamate. The supply not only of glutamate, but also of glutamine, and the reaction of synthesizing glutamate from glutamine, has most likely reached a peak in this condition. Under these circumstances, it appears difficult to increase glutamine taking from blood even further to compensate for the decreased availability of glutamate. If the glomeruli that act as filters decrease in number, causing a drop in eGFR, and if the RPTs’ glutamate reabsorptive function declines, it would become difficult to fulfil the RPTs’ demand for glutamate which has now increased because of increased glutathione synthesis, etc. (Figures 2 and 3). We presume that the human body tries to cancel this glutamate shortage by raising the blood level of glutamine which can be taken directly from blood but not glutamate which cannot rise in the reabsorption. Blood glutamine concentration can be increased by the hepatic glutamine release, and direct taking of glutamine from the blood into the RPT is possible. In contrast, glutamate cannot be taken directly from the blood [19]. The hepatic production of glutamate increases at the gluconeogenesis accentuation (Alanine → pyruvate+glutamate). This reaction is catalysed by glutamate-pyruvate transaminase (GPT) that doesn't exist in the kidney. Under this condition, supply from the liver to the blood may shift predominantly from glutamate to glutamine, which can be taken from the blood. In the state of emergency such as diabetes and starvation, pyruvate and glutamate is synthesized from alanine in the liver, in addition the glutamate is converted into glutamine, and the glutamine is supplied into blood (Figure 3 and Supplementary Figure 1) [29-31]. Our results showed that a reduction in eGFR correlated strongly with a reduction in the reabsorption of glutamate, and a reduction in HbA1c and GA also correlated positively with a reduction in the reabsorption of glutamate. Therefore, a reduction in the reabsorption of glutamate is closely correlated to a decline in renal function, and we believe is also related to decreased glycaemia. However, it is uncertain whether the eGFR decrease is a cause of the glutamate reabsorption decrease or the glutamate reabsorption decrease is a cause of the eGFR decrease [24-26]. If the number of nephrons decreases, in order to maintain the total amount of renal gluconeogenesis, it is necessary to increase the rate of gluconeogenesis per residual nephron (Supplementary Figure 2). Since the reabsorption of glutamate is already close to 100% and cannot be increased any further, it is virtually impossible to increase the supply of glutamate per nephron. This is compensated for by increasing glutamine taken from the blood, but if the nephron count decreases to a level that cannot be compensated for, there is a possibility that renal gluconeogenesis may decrease and that blood glucose levels will decline (Supplementary Figure 3). The RPTs perform gluconeogenesis and supply glucose to the blood. The substrates for this renal gluconeogenesis are lactate, glycerol, and glutamine in the blood [4]. Lactate is converted into pyruvate inside the cytosol, and enters the mitochondria, where they become oxaloacetate (Figure 3). Pyruvate carboxylase, an enzyme that converts pyruvate into oxaloacetate, is not present inside the cytosol and exists only inside the mitochondria [32]. Therefore, oxaloacetate cannot be synthesized within the cytosol. After glutamine becomes glutamate, it turns into α-ketoglutarate, enters the TCA cycle, and becomes oxaloacetate (Figures 2 and 3) [19]. In gluconeogenesis, glucose is made from phosphoenol pyruvate (PEP). The PEP is produced by the decarboxylation reaction, which is catalysed by phosphoenolpyruvate carboxykinase (PEPCK), which uses oxaloacetate in the cytosol as its substrate (Figure 3). Bicarbonate (HCO3 -) is synthesized using this carbon dioxide (CO2) and used for adjusting the blood pH (Figure 2) [19]. Oxaloacetate in the cytosol is obtained by transporting, to the cytosol, the oxaloacetate that has been synthesized in the mitochondria. However, since oxaloacetate cannot pass through the mitochondrial membrane, it is converted into either malate or asparagine, which is then transported to in the cytosol, and later re-converted into oxaloacetate. This is the “malateasparagine shuttle” (Figure 4) [14].
| Control | Diabetes | P | |||||
|---|---|---|---|---|---|---|---|
| 100 | 100 | ||||||
| Plasma (µmol/dL) | |||||||
| Glutamate | 259.9 | ± | 89.8 | 62.6 | ± | 20.3 | <0.01 |
| Glutamine | 286.5 | ± | 95.0 | 530.6 | ± | 78.3 | <0.01 |
| Cysteine | 1.3 | ± | 0.5 | 16.9 | ± | 15.6 | <0.01 |
| Urinary (µmol/g Cre) | |||||||
| Glutamate | 19.3 | ± | 8.6 | 34.3 | ± | 27.8 | <0.01 |
| Glutamine | 302.5 | ± | 138.5 | 424.7 | ± | 352.2 | <0.01 |
| Cysteine | 49.6 | ± | 24.6 | 109.1 | ± | 131.4 | <0.01 |
| Reabsorption (%) | |||||||
| Glutamate | 99.93 | ± | 0.04 | 99.42 | ± | 0.78 | <0.01 |
| Glutamine | 99.03 | ± | 0.61 | 99.13 | ± | 1.61 | 0.58 |
| Cysteine | 70.53 | ± | 11.60 | 88.09 | ± | 16.97 | <0.01 |
Control: Control non diabetic obese young subjects; Mean ± SD
Table 6: A comparison of the blood glutamate, glutamine and cysteines levels and the urinary the amino acids excretion between the control group and the diabetic group.
Figure 4: Overview of the malate-aspartate shuttle. An oxaloacetate is synthesized only in the mitochondria. An oxaloacetate in the cytosol is necessary for gluconeogeknesis. However, the oxaloacetate is not synthesized in the cytosol. Then, it is necessary that the mitochondrial oxaloacetate is moved into the cytosol. However, oxaloacetate cannot pass the mitochondrial membrane. After oxaloacetate is converted into malate or aspartate that can pass the mitochondrial membrane and they are passed, they are reconverted to oxaloacetate in cytosol. A glutamate is indispensable to transport the oxaloacetate for gluconeogenesis.
In the reaction that synthesizes pyruvate from lactate, NAD+ is required as a coenzyme; however, since the only reaction that can produce NAD+ in the cytosol is the reaction that produces malate from oxaloacetate, this reaction is inclined towards the synthesis of malate. It is therefore difficult to transport oxaloacetate using malate. Asparagine is therefore employed for transporting oxaloacetate. Here, glutamate is essential for powering this shuttle (Figure 4 and Supplementation of Figure 4).
Limitations
Our study has not evaluated renal gluconeogenesis or renal glucose release, so no definitive results have been obtained on the relationship between these amino acids metabolisms and renal gluconeogenesis. There is a need to harvest and compare renal arterial blood (blood prior to being processed at the kidney) and renal venous blood (blood after having been processed at the kidney) (Supplementary Figure 4) [33]. It is difficult, however, to increase the number of subjects available for this sampling, so, in our study, we focused instead on increasing the number of subjects. The relationship between the reduction in glutamate reabsorption and the reduction in renal gluconeogenesis has therefore not been proven in this study. Moreover, the phenomenon of BGCs dropping because of renal dysfunction is an already-known fact; however, whether or not this is due to a decrease in renal gluconeogenesis remains unknown. We report this study in the expectation that the results of our research can contribute to elucidating these questions.
Disclosures
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors wish to thank Miss Keiko Kawamura and nurses for their efforts in gathering subject data. Our deep appreciation goes to all staff members who helped us in this study.
Funding Information
This work was supported by Tohoku University's Center for the Advancement of Higher Education President's Research Fund.
Conflict of Interest Disclosure Statement
All authors declare no conflicts of interest.
References
- Gosmanov AR, Gosmanova EO, Kovesdy CP (2016) Evaluation and management of diabetic and non-diabetic hypoglycemia in end-stage renal disease. Nephrol Dial Transplant 31: 8-15.
- O'Brien JP, Sharpe AR Jr (1965) Abnormal Carbohydrate Metabolism in Renal Failure. Metabolism 14: 1294-1306.
- Duckworth WC (1988) Insulin degradation: mechanisms, products, and significance. Endocr Rev 9: 319-345.
- Gerich JE, Meyer C, Woerle HJ, Stumvoll M (2001) Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24: 382-391.
- Stumvoll M, Meyer C, Mitrakou A, Gerich JE (1999) Important role of the kidney in human carbohydrate metabolism. Med Hypotheses 52:363-366.
- Mitrakou A (2011) Kidney: its impact on glucose homeostasis and hormonal regulation. Diabetes Res Clin Pract Suppl 1: S66-72.
- Arem R (1989) Hypoglycemia associated with renal failure. Endocrinol Metab Clin North Am 18: 103-121.
- Garibotto G, Sofia A, Saffioti S, Bonanni A, Mannucci I, et al. (2010) Amino acid and protein metabolism in the human kidney and in patients with chronic kidney disease. Clin Nutr 29: 424-433.
- Tada K, Hirono H, Arakawa T (1967) Endogenous renal clearance rates of free amino acids in prolinuric and Hartnup patients. Tohoku J Exp Med 93: 57-61.
- Ogawa S, Takiguchi J, Shimizu M, Nako K, Okamura M, et al. (2016) The Reduction in Urinary Glutamate Excretion Is Responsible for Lowering Urinary pH in Pink Urine Syndrome. Tohoku J Exp Med 239: 103-110.
- Ogawa S, Takiguchi J, Shimizu M, Nako K, Okamura M, et al. (2017) The relationship between the renal reabsorption of cysteine and the lowered urinary pH in diabetics. Clin Exp Nephrol 21: 1044-1052.
- Ogawa S, Takiguchi J, Nako K, Okamura M, Sakamoto T, et al. (2015) Elucidation of the etiology and characteristics of pink urine in young healthy subjects. Clin Exp Nephrol 19: 822-829.
- Welbourne TC, Horton K, Cronin MJ (1992) Growth hormone and renal glutamine and glutamate handling. J Am Soc Nephrol 2: 1171-1177.
- Darvey IG (2000) Does the transport of oxaloacetate across the inner mitochondrial membrane during gluconeogenesis require carrier proteins other than those used in the malate-aspartate shuttle? Biochem Educ 28: 80-82.
- Winiarska K, Bozko P, Lietz T, BryÃÆââ¬Â¦Ãâââ¬Å¡a J (1998) Importance of glutamate dehydrogenase stimulation for glucose and glutamine synthesis in rabbit renal tubules incubated with various amino acids. Acta Biochim Pol 45: 825-831.
- Lietz T, Winiarska K, BryÃÆââ¬Â¦Ãâââ¬Å¡a J (1997) Ketone bodies activate gluconeogenesis in isolated rabbit renal cortical tubules incubated in the presence of amino acids and glycerol. Acta Biochim Pol 44: 323-331.
- Fernandez CA, Des Rosiers C (1995) Modeling of liver citric acid cycle and gluconeogenesis based on 13C mass isotopomer distribution analysis of intermediates. J Biol Chem 270: 10037-10042.
- Kunz WS, Davis EJ (1991) Control of reversible intracellular transfer of reducing potential. Arch Biochem Biophys 284: 40-46.
- Hediger MA (1999) Glutamate transporters in kidney and brain. Am J Physiol 277:F487-492.
- Lash LH, Tokarz JJ (1990) Oxidative stress in isolated rat renal proximal and distal tubular cells. Am J Physiol 259: F338-347.
- Visarius TM, Putt DA, Schare JM, Pegouske DM, Lash LH (1996) Pathways of glutathione metabolism and transport in isolated proximal tubular cells from rat kidney. Biochem Pharmacol 52: 259-272.
- Shayakul C, Kanai Y, Lee WS, Brown D, Rothstein JD, et al. (1997) Localization of the high affinity glutamate transporter EAAC1 in rat kidney. Am J Physiol 273: F1023-1029.
- Tietze IN, Sørensen SS, Eiskjaer H, Thomsen K, Pedersen EB (1992) Tubular handling of amino acids after intravenous infusion of amino acids in healthy humans. Nephrol Dial Transplant 7: 493-500.
- Mahieu S, Klug M, Millen N, Fabro A, Benmelej A, et al. (2016) Monosodium glutamate intake affect the function of the kidney through NMDA receptor. Life Sci 149: 114-119.
- Dryer SE (2015) Glutamate receptors in the kidney. Nephrol Dial Transplant 30: 1630-1638.
- Epstein FH, Brosnan JT, Tange JD, Ross BD (1982) Improved function with amino acids in the isolated perfused kidney. Am J Physiol 243: F284-292.
- Silverman M, Vinay P, Shinobu L, Gougoux A, Lemieux G (1981) Luminal and antiluminal transport of glutamine in dog kidney: effect of metabolic acidosis. Kidney Int 20:359-365.
- Burch HB, Chan AW, Alvey TR, Lowry OH (1978) Localization of glutamine accumulation and tubular reabsorption in rat nephron. Kidney Int 14: 406-413.
- Blackshear PJ, Holloway PA, Alberti KG (1975) Factors regulating amino acid release from extra splanchnic tissues in the rat. Interactions of alanine and glutamine. Biochem J 150: 379-387.
- Botini FF, Suzuki-Kemmelmeier F, Nascimento EA, Ide LT, Bracht A (2005) Zonation of alanine metabolism in the bivascularly perfused rat liver. Liver Int 25: 861-871.
- Brosnan JT (2000) Glutamate, at the interface between amino acid and carbohydrate metabolism. J Nutr 130: 988S-990S.
- Jitrapakdee S, Vidal-Puig A, Wallace JC (2006) Anaplerotic roles of pyruvate carboxylase in mammalian tissues. Cell Mol Life Sci 63: 843-854.
- Stumvoll M, Meyer C, Mitrakou A, Nadkarni V, Gerich JE (1997) Renal glucose production and utilization: new aspects in humans. Diabetologia 40: 749-757.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences