Cyclosporine-induced Hemolytic Uremic Syndrome Following ABO Incompatible Living Donor Renal Transplantation: Case Report
Koichi Kozaki, Kentaro Tani and Kenji Yuzawa
DOI10.21767/2472-5056.100028
Koichi Kozaki1,2*, Kentaro Tani3 and Kenji Yuzawa2
1Department of Surgery, National Hospital Organization Mito Medical Center, Ibaraki, Japan
2Transplantation Surgery, National Hospital Organization Mito Medical Center, Ibaraki, Japan
3Department of Pharmacy, National Hospital Organization Mito Medical Center, Ibaraki, Japan
- *Corresponding Author:
- Koichi Kozaki
Department of Surgery, National Hospital Organization Mito Medical Center
Ibaraki, ZIP 311-3193, Japan
Tel: 81-29-240-771
E-mail: k.kozaki.d@mn.hosp.go.jp
Received date: January 20, 2017; Accepted date: February 01, 2017; Published date: February 03, 2017
Citation: Kozaki K, Tani K, Yuzawa K (2017) Cyclosporine-induced Hemolytic Uremic Syndrome Following ABO Incompatible Living Donor Renal Transplantation-Case Report. J Clin Exp Nephrol 1:28. DOI: 10.21767/2472-5056.100028
Copyright: © 2017 Kozaki K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
We experienced a case with suspected drug-induced Hemolytic Uremic Syndrome (HUS) by immunosuppressive drug cyclosporine (CYA). HUS developed in a 51-year- old woman treated with CYA after ABO blood type Incompatible Living Donor Renal Transplant (ILDRTx). Her blood type was A positive, underwent ILDRTx from her husband whose blood type was B positive. She had developed hemolytic anemia with erythrocyte fragmentation, severe thrombocytopenia and acute renal failure on the first day after ILDRTx. It was thought with CYA-induced HUS not ABO blood type antibody mediated rejection at that point because the ABO blood type antibody titers did not rise, and there were no renal graft blood flow decreases in ultrasonography and renography. On day 2 after ILDRTx, CYA was converted to tacrolimus. After conversion without therapy for rejection, HUS was gradually improved. On day 20, she was discharged from our hospital with a serum creatinine (S-Cr) level of 1.65 mg/dl, and S-Cr level was 0.86 mg/dl six years and seven months after discharge. CYAinduced HUS is extremely rare with less than 1%, but serious complication of CYA therapy resulting in graft loss in many cases. Therefore early detection, early treatment is important.
Hemolytic uremic syndrome (HUS) is characterized clinically by hemolytic anemia, thrombocytopenia, and acute renal failure. At ABO blood type incompatible living donor renal transplantation (ILDRTx), it is well known to produce HUS with ABO blood type antibody mediated rejection. Whereas it is a rare cause, but cyclosporine (CYA) is one of the causes of HUS after the organ transplantation, and attention is necessary when we use CYA. We report herein a case of CYA-induced HUS, successfully managed by discontinuance CYA, and conversion to tacrolimus (TAC).
Case Report
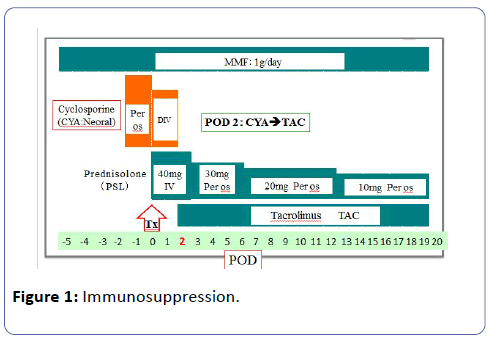
A 51-year-old woman who suffered from chronic renal failure caused by chronic glomerulonephritis, and had been treated by hemodialysis (HD) for ten years. In June 2010, she, whose blood group was A positive, underwent ILDRTx from her 52-year-old husband whose blood type B positive. HLA type was onemismatch, and lymphocytes cross-match test was negative. For desensitization, we underwent laparoscopic splenectomy two weeks before transplantation (Tx), and performed twice of double filtration plasmapheresis preoperatively. We reduced the titer of anti-B antibody (Ab) to IgM/IgG=32/<2 by desensitization, and underwent ILDRTx subsequently. CYA, prednisolone and mycophenolate mofetil (MMF) were administered for immunosuppression. She initially received combination immunosuppressive therapy consisting of CYA and MMF starting two days prior to Tx to our immunosuppressive regimen (Figure 1). Briefly, an initial oral dose of CYA (6 mg/kg) was given twice a day for two days prior to Tx. A three hours intravenous infusion of 2 mg/day per day was administered twice for the first two days after Tx. The daily CYA dose was adjusted according to the following control, the whole blood target trough level being 200-250 ng/ml up to 2 weeks, 150-200 ng/ml up to 4 weeks and less than 150 ng/ml thereafter.
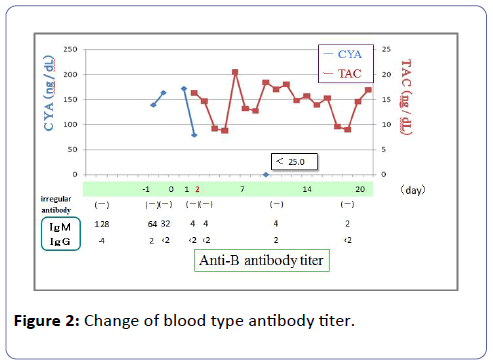
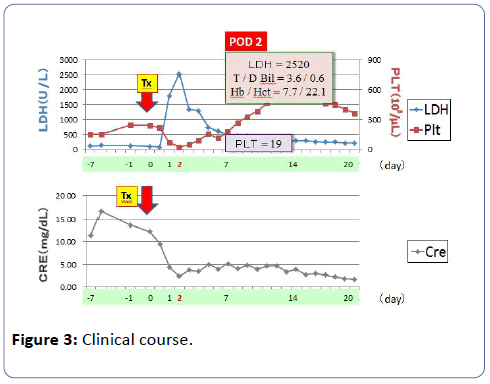
The graft functioned immediately and the serum creatinine (S-Cr) level decreased from 9.49 mg/dl to 4.30 mg/dl on postoperative day 1. Although the graft function promptly recovered by day 1, urine output has begun to gradually decrease from 150 ml/hr or more to 30-40 ml/hr, and subsequently the patient became anuric at midnight on day 1 (on day 3 CYA administration). From day 2, because renal function deteriorated progressively, the patient required HD. From day 3, the patient fell into acute tubular necrosis (ATN) clinically. As for the patients, there was no ultrasonographic and renographic evidence of renal artery stenosis or obstructive uropathy although the patient suffered from ATN. Laboratory findings on day 1 disclosed : hemoglobin (Hb) 7.4 g/dl (10.8 g/dl at day 1 after Tx); hematocrit 22.1% (32.0%); platelet count (PLT) 37 × 103/μl (134 × 103/μl); total bilirubin (TD) 2.2 mg/dl; lactate dehydrogenase (LDH) 1133 IU/ml (86 IU/ml); S-Cr 6.25 mg/dl (8.30 mg/dl) on day 1 after Tx. As laboratory studies revealed a Coombs-negative microangiopathic hemolytic anemia. Fragmented erythrocytes were found in a peripheral blood smear. There was no clinical evidence of infection. Because the patient took aspirin under the anticoagulant therapy, percutaneous renal graft biopsy was not performed. Thus, it was thought with HUS from haemolytic anemia, thrombocytopia, and acute renal failure. Anti-B Ab titer did not rise, such as IgM/ IgG=4/<2, and subsequently it did not rise either (Figure 2). From the above-mentioned results, we had a diagnosis of CYAinduced HUS. On the postoperative day 2, CYA treatment was discontinued and TAC was added to the immunosuppressive regimen. Packed red blood cells infusion, platelet concentrate infusion without using fresh frozen plasma infusion and HD were performed from day 1 after Tx. From the first to fourteenth day, the patient received 16 units (2240 ml) of packed red blood cells. From the first to forth day 75 units of platelet concentrate were transfused. PLT increased gradually, and on the seventh day PLT was 179 × 103/μl, Hb value was 9.8 g/dl and laboratory tests suggested a remission of the hemolysis. On the thirteenth day renal graft function began to improve progressively, the urine output was gradually increasing. On the fourteenth day urine output was 575 ml/day, and HD was stopped. On the twentieth day S-Cr level dropped to 1.65 mg/dl, and the patient was discharged from our hospital. One year and three months after Tx, her S-Cr level was 1.13 mg/dl (Figure 3), and do not show the recurrence of the HUS under TAC treatment with LDH 142 IU/L, TD=0.4 mg/dl, Hb/Hct=10.6 g/dl /32.7%, platelet count 298 × 103/μl. To date, no relapse of HUS occurred under TAC therapy.
Discussion
Drug-induced HUS defined by microangiopathic hemolytic anemia [1,2]. The clinical features of patients reported to have drug-induced HUS suggest diverse mechanisms of adverse drug reaction [3]. Al-Nouri et al. reviewed publicshed reports of druginduced HUS.3) According to this report, published data describe 78 drugs suspected of causing drug-induced HUS and only 22 (28%) of the 78 drugs have evidence supporting a definite causal association with drug-induced HUS [4]. As would be expected, immunosuppresant including cyclosporine (CYA), tacrolimus (TAC) and everolimus was the culpable agent.
Our patient fulfilled the criteria for HUS as first reported by Gasser et al. including Coombs-negative microangiopathic hemolytic anemia, thrombocytopenia and acute renal failure [5]. The causes of HUS are: 1) unknown etiology, 2) infection (E. coli O-157, Shigella), 3) drugs (CYA, cisplatin, mitomycin C), 4) collagen disease (SLE, PSS), and 5) other (radiation, pregnancy delivery). Therefore, a variety of therapy has been proposed for HUS, such as steroids, heparin, urokinase, antiplatelet drugs, splenctomy and plasma therapy (plasma exchange or plasma infusion without plasma exchange), but there is not the established method [6-10]. We present herein a case of posttransplant HUS induced by CYA. There have been many reports of renal transplant recipients in whom CYA-induced HUS developed after Tx that was potentially reversible by discontinuance of CYA [11-13]. Our patient fulfilled typical triad for HUS, such as hemolytic anemia, thrombocytopenia and acute renal failure. This patient was administered CYA, and despite low CYA trough levels (approximately 150 ng/ml in whole blood), HUS developed in the patient. Therefore, the onset of the HUS is thought to have possibilities to be caused by vascular endothelium injury by CYA administration. Whereas there is the report that CYA concentration is associated with for the onset of the CYA-induced HUS.
The exact mechanism of CYA-induced HUS, although still unknown, involves endothelial cell toxicity by CYA, which seems to be dose dependent [14]. If this hypothesis is correct, the therapy of CYA-induced HUS is withdrawal or dose reduction of CYA. CYA reinforces vasoconstrictive factors such as thromboxane or endothelin, and it is said that CYA inhibit the production of vasodilatation factors such as prostacycline or nitric oxide at the same time and thereby causes the vasoconstriction of the afferent and efferent glomerular arterioles, and it is said that vascular endothelial cells are affected, and HUS develops [15-18]. CYA inhibits prostacycline (PGI2) composition, reported by Leithner et al. [19]. As a result, it causes HUS. Remuzzi and Maclntyre et al. [8,20]. Reported that CYA have decreased prostacycline stimulating factor (PSF) in the patients, and deficiency of PSF causes the PGI2-producing disorder of endothelial cells, as a result, it causes HUS. Hirose et al. [21] reported that CYA affected the size of red cells and directly produced hemolysis in an isotonic buffer without the involvement of an immune mechanism, and superoxide (SO) and CYA showed a synergistic effect on hemolysis during prolonged incubation. In that SO is produced in excess during the recovery of blood flow after Tx, the prolonged contact of red cells with CYA and SO may be involved in the development and reinforcement of hemolysis in vivo.
In the case of the HUS that occurred under CYA treatment, we undergo withdrawal or dose reduction of CYA. Another treatment is replacement of CYA with TAC, reported by McCauley et al. [22]. Our case of CYA-induced HUS, successfully managed by discontinuance CYA, and conversion to TAC without any complication such as rejection and TAC-induced HUS. By the way, there are fewer reports of the HUS onset by the TAC that is same calcineurin inhibitors (CNI) than CYA. According to Abraham et al. [23] the post-transplant HUS incidence is assumed 3-5% in CYA and 1% in TAC. However, TAC has only been in clinical use for a relatively short period of time and that comparisons with CYA in this respect may not be valid. Therefore, we must always take into consideration that the HUS occurs in not only CYA but also TAC.
Post-transplant HUS has a poor prognosis with a high incidence of graft loss and mortality, namely 50% and 5%, respectively [6]. In conclusion, because of a high incidence of graft loss and mortality, early diagnosis seems very useful and important in the management of CYA or TAC-induced HUS along with discontinuance of CYA or TAC.
References
- George JN, Nester CM (2014) Syndromes of thrombotic microangiopathy. N Engl J Med 371: 654-666.
- Noris M, Remuzzi G (2010) Thrombotic microangiopathy after kidney transplantation. Am J Trans 10: 1517-1523.
- Edwards IR, Aronson JK (2000) Adverse drug reactions: definitions, diagnosis, and management. Lancet 356: 1255-1259.
- Al-Nouri ZL, Reese JA, Terrell DR, Vesely SK, George JN (2015) Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood 125: 616-618.
- Gasser C, Gautier E, Steck A, Siebenmann RE, Oechslin R (1955) Hemolytic-uremic syndrome: bilateral necrosis of the renal cortex in acute acquired hemolytic anemia. Schweiz Med Wochenschr 85: 905-909.
- Wolyniec W, DÃÆââ¬Å¾Ãâââ¢bska-ÃÆââ¬Â¦Ãâà ¡lizieÃÆââ¬Â¦Ãâââ¬Å¾, Chamienia A, Ignacy Pirski M, ÃÆââ¬Â¦ÃâÃÂysiak-SzydÃÆââ¬Â¦Ãâââ¬Å¡owska W, et al. (2002) Cyclosporine A-related hemolytic uremic syndrome after living renal transplantation-Case report. Transplant Proc 34: 569-571.
- Asaka M, Ishikawa I, Nakazawa T, Tomosugi N, Yuri T, et al. (2000) Hemolytic uremic syndrome associated with influenza A virus infection in an adult renal allograft recipient: case report and review of the literature. Nephron 84: 258-266.
- Remuzzi G (1996) Role of endothelin in the development of glomerulosclerosis. Kidney Blood Press Res 19: 182-183.
- Kulzer P, Wanner C (1998) Thrombotic microangiopathy: a challenge with uncertain outcome. Nephrol Dial Transplant 13: 2154-2160.
- Hayward CP, Sutton DM, Carter WH, Campbell ED, Scott JG, et al. (1994) Treatment outcomes in patients with adult thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Arch Intern Med 154: 982-987.
- Van Buren D, Van Buren CT, Flechner SM, Maddox AM, Verani R et al. (1985) De novo hemolytic uremic syndrome in renal transplant recipients immunosuppressed with cyclosporine. Surgery 98: 54-62.
- Wolfe JA, McCann RL, Sanfilippo F (1986) Cyclosporin associated microangiopathy in renal transplantation: A severe but potentially reversible form of early graft injury. Transplantation 41: 541-543.
- Hochstetler LA, Flanigan MJ, Lager DJ (1994) Transplant-associated thrombotic microangiopathy: the role of IgG administration as initial therapy. Am J Kidney Dis 3: 444-450.
- Myers BD (1986) Cyclosporine nephrotoxicity. Kidney Int 30: 964-974.
- Brown Z, Neild GH (1987) Cyclosporine inhibits prostacyclin production by cultured human endothelial cells. Transplant Proc 19: 1178-1180.
- Moake JL (1994) Haemolytic-uraemic syndrome: basic science. Lancet 343: 393-397.
- Textor SC, Burnett JC, Romero JC, Canzanello VJ, Taler SJ, et al. (1995) Urinary endothelin and renal vasoconstriction with cyclosporine or FK506 after liver transplantation. Kidney Int 47: 1426-1433.
- Zoja C, Furci L, Ghilardi F, Zilio P, Benigni A (1986) Cyclosporin-induced endothelin cell injury. Lab Invest 55: 455-461.
- Leithner C, Sinzenger H, Pohanka E, Schwarz M, Kretschmer G, et al. (1983) Occurrence of hemolytic uremic syndrome under cyclosporine treatment: Accident or possible side effect mediated by a lack of prostacyclin stimulating plasma factor ? Transplant Proc 15: 2787-2789.
- Maclntyre DE, Pearson JD, Gordon JL (1978) Localization and stimulation of prostacyclin production on vascular cell. Nature 271: 549-551.
- Hirose M, Kuroda Y (1994) Reinforcement of cyclosporine A-induced red cell destruction by superoxide. Tokushima J exp Med 41: 65-70.
- McCauley J, Bronsther O, Fung J, Todo S, Starzl T (1989) Treatment of cyclosporine-induced haemolytic uraemic syndrome with FK506. Lancet 2: 1516.
- Abraham KA, Little MA, Dorman AM, Walshe JJ (2000) Hemolytic uremic syndrome in association with both cyclosporine and tacrolimus. Transpl Int 13: 443-447.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences