Curcumin Promotes Anti-Tumour Effect of Cisplatin and Protects against CisplatinInduced Kidney Injury in Lung Cancer
Miaoxin Zhao, Yue Ma, Xiangka Hu, Yuqi Sun, Zuodong Liu, Sumin Dong, Hua Liu and Chunmei Dai*
DOI10.36648/2472-5056.21.s3.007
Miaoxin Zhao, Yue Ma, Xiangka Hu, Yuqi Sun, Zuodong Liu, Sumin Dong, Hua Liu and Chunmei Dai*
College of Pharmacy, Jinzhou Medical University, Liaoning Province, China
- *Corresponding Author:
- Chunmei Dai
College of pharmacy,
Jinzhou Medical University,
Liaoning Province,
China.
E-mail: lnmupharmacy@163.com.
Received Date: July 29, 2021;Accepted Date: August 12, 2021;Published Date: August 19, 2021
Citation: Zhao M, Ma Y, Hu X, Sun Y, Dai C, et al. (2021) Curcumin Promotes Anti- Tumour Effect of Cisplatin and Protects against Cisplatin-Induced Kidney Injury in Lung Cancer. J Clin Exp Nephrol Vol. 6 No.S3: 007.
Abstract
Background: Lung cancer patients will develop strong drug resistance and serious complications, especially severe renal injuries, after long-term treatment of platinum-based drugs. Curcumin (Cur) combined with cisplatin (DDP) has a synergistic and attenuating effect on lung cancer. However, the role of curcumin in protection against DDP kidney injury in lung cancer remains unknown.
Methods: We conducted in vitro experiments to evaluate the anti-tumour effect of cur combined with DDP such as MTT, mitochondrial membrane potential (ΔΨm) analysis, and flow cytometry. The serology and histopathology were used to assess kidney injury induced by DDP in vivo experiments. Western blotting analysis was utilized to detect P38 levels in mouse kidneys.
Results: A significant synergistic antitumor effect in A549 and LLC-1 cells based on the activation of the mitochondrial apoptosis pathway. Moreover, Cur could reduce the nephrotoxicity of DDP treatment through the P38MAPK signalling pathway.
Conclusion: Cur in combination with DDP may be considered as a beneficial combination regimen for enhancing DDP sensitivity and providing a promising Reno protective effect against nephrotoxicity induced by DDP.
Keywords
Curcumin; Cisplatin; Lung cancer; Apoptosis; Nephrotoxicity; P38MAPK
Introduction
Lung cancer is the commonest cancer. It ranks first in terms of mortality from cancer in men and ranks second in women [1]. Chemotherapy and radiotherapy are necessary for treatment, but they can cause severe side effects, which is one reason that developing new therapies is required to improve existing treatment[2,3]. Cisplatin (cis-diamminedichloroplatinum II) is a potent chemotherapeutic agent for treating various solid-organ cancers [4]. Clinical researchers have identified renal toxicity as the main side effect of cisplatin at a limited dose [5,6]. Cisplatin is a non-organic platinum-containing compound with alkylating properties. It causes cross-linking of DNA and RNA chains [7,8]. Cisplatin has also been suspected to generate reactive oxygen species (ROS), which have also been linked to direct cellular damage [9]. Superoxide anion, hydroxyl radical, and hydrogen peroxide are the causes of oxidative stress renal injury induced by cisplatin [10]. In addition, enhanced lipid peroxidation and inhibition of antioxidant defenses are signs of cisplatin-induced nephrotoxicity [11].
In recent years, it has been shown that combined chemotherapy can improve treatment results and/or reduce the side effects of standard drug regimens. For example, when irinotecan and sunitinib are used in combination in vivo and in vitro, they have a significant synergistic anti-tumor effect on anaplastic thyroid carcinoma [12]. Some results have demonstrated that combined treatment with polysaccharides can enhance the efficacy of DDP and reduce chemotherapy-induced adverse reactions [13]. For example, combination treatment with Oridonin can enhance the antitumor efficacy of adriamycin in the treatment of invasive breast cancer through stimulating apoptotic and anti- angiogenesis [14]. Therefore, combination treatment is hopeful to be a prospective and valid anti-tumour treatment tactics.
In this study, the anti-tumour activity of Cur combined with DDP was investigated in vitro and vivo. Besides, we found that combined Cur treatment reduced the nephrotoxicity caused by DDP and further explored the related molecular mechanisms. This research will help develop new combined chemotherapy strategies to achieve the goal of enhancing efficacy while reducing the nephrotoxicity of lung cancer patients.
Materials and Methods
Materials and reagents
The human lung adenocarcinoma cell line A549 and the mouse cell line LLC-1 were obtained from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Cisplatin (DDP) was acquired from Jiangsu Haoseng Pharmacuetics Co., Ltd. (Lot number 181001; Jiangsu, China). Fetal bovine serum (FBS), high-glucose DMEM, trypsin- EDTA, penicillin and streptomycin were bought from Gibco (Grand Island, NY, USA). Curcumin (Cur) was purchased from Sigma-Aldrich and was stored at JinZhou Medical University of Technology until use. Test kits glutathione (GSH) and malondialdehyde (MDA) as well as the mitochondrial membrane potential assay kit of JC-1 were all bought from Beyotime Institute (Shanghai, China). Annexin V-FITC Apoptosis Detection Kit was obtained from Vazyme Biotech Co.,Ltd. (Nanjing, China).
Cell lines
The human lung adenocarcinoma cell line A549 and the Lewis lung cancer cell line were cultured in DMEM added 10% FBS, 100 U/mL of penicillin and 100 μg/mL of streptomycin (complete medium). Replace the complete medium every two days.
Cell viability assay
Cell survival rate was measured through MTT analysis. In simple terms, logarithmic growth A549 and LLC-1 cells were slaked with 0.25% trypsin, added to DMEM containing 10% fetal bovine serum and eventually plated on 96-well plates at a density of 0.8 × 104 cells/well. After hatch for 24 h, DDP was combined with different concentrations of Cur. Next, MTT (5 mg/mL, 10 μL) reagent was joined and incubated at 37 °C for 4 h. Then, the supernatant was abandoned, and dimethyl sulfoxide (DMSO, 150 μL) was joined to each well. The OD value was obtained at 490 nm by Spectra Max 190 micrometre. The OD (optical density) of each hole was in comparison with the normal group treated with DMSO alone. The measurements should be made three times.
Annexin-V/PI staining
The Annexin V-FITC Apoptosis Assay Kit was used to assess the percentage of apoptotic/necrotic cells. The cells were suspended in 110 μL of 1 × Binding Buffer containing FITC-labeled Annexin and propidium iodide (kit provided). After incubation for 10 min, 400 μL of 1 × Binding Buffer was added to the samples, which were analyzed in a BD FACSCalibur™ cytometer using the FL1-H (FITC) and FL3-H (PI) channels. Appropriate staining controls were used to calibrate the blood cell counter. FLOWJO® software was used to allocate and quantize living cells (Annexin V-/PI-), early apoptotic cells (Annexin V+/PI-), late apoptotic ells (Annexin V+/ PI+) and necrotic cells (Annexin V-/PI+).
Analysis of ΔΨm
The ΔΨm was tested by a ΔΨm detection kit (Beyotime). ΔΨm transformation can be mirrored by the ratio of green to red fluorescence. The green/red fluorescence ratio was used for estimating chondriosome unpolarizing.
Lung tumour-bearing model in mouse and its treatments
Forty-eight C57BL/6 male mice, weighing about 20 ± 2 g, were bought from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. (Certificate number: SCXK 2006-0006, Beijing, China). After they were cultivated in medium at about 4 d, LLC-1 cells were adjusted to a density of 1 × 107 cell/mL with sterile PBS to product a cell suspension. Then, the cell suspension was subcutaneously injected to the right armpits of forty-eight healthy mice at a dose of 0.2 mL each mouse. After tumors growing about 4 d, tumors with a dimension of approximately 0.5 cm were deemed a sign of a successfully established model. Mice in the normal group were treated with saline, DDP (5 mg/ kg), DDP plus Cur L (5 mg/kg+50 mg/kg), DDP plus Cur M (5 mg/ kg+100 mg/kg), DDP plus Cur H (5 mg/kg+200 mg/kg) or DDP plus SB203580 (5 mg/kg+4 mg/kg). Cur was administered by intragastric administration once a day, while DDP were given through intraperitoneal every two days and SB203580 were given through intraperitoneal every three days; treatment with a daily injection began immediately and lasted 14 days when the models were built successfully.
Biochemical indicators and enzyme-linked immunosorbent assay
The GSH and MDA were assessed using an ELISA kit.
Western blotting analysis
total protein concentration was quantified by a BCA protein assay kit (Applygen, China). The proteins (100 μg) were separated by electrophoresis and transferred onto PVDF membranes (Thermo Fisher Scientific, USA). Actin (1:3000, ab8227, Abcam) served as a reference gene.
Histology staining
After fixation in formaldehyde solution for 48 h, the tissues were embedded in paraffin, and sectioned into slices (5 μm thick). The slices were stained with hematoxylin-eosin (H and E) (Phygene Life Sciences Co., Ltd, Fuzhou, China), next observation under a microscope at 400 × magnification.
Statistical analyses
After three independent experiments were conducted, the data were appeared as the means ± SD (standard deviation). Statistical analyses were carried out with Student’s t-test by a software package (SPSS 23.0, Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
Curcumin promotes cytotoxicity of cisplatin in A549 and LLC-1 cells in vitro
The cytotoxicity of DDP and Cur in A549 and LLC-1 cells was evaluated using MTT analysis (Figure 1). We used Cur alone to treat two lung cancer cell lines, and the results showed a concentration-dependent decrease in cell viability (Supplementary Figure 1A). And our data showed that the IC50 values of Cur in A549 and LLC-1 cells were 58.21 and 34.68μM, respectively (Supplementary Figure 1B). To identify whether Cur could synergize the cytotoxicity of DDP, we identified the dosage of DDP as 4μg/ml (Supplementary Figure 1C) and the doses of Cur as 10, 20, 40, and 80 μM (Supplementary Figure 1B). As shown in Supplementary Figure 1D we clearly found that after DDP was combined with different concentrations of Cur, the viability of A549 or LLC-1 cells was significantly decreased and significantly inferior to that of cells treated with DDP alone. Thus, our data indicated that Cur potentiated the anti-tumor effect of DDP in A549 and LLC-1 cells.
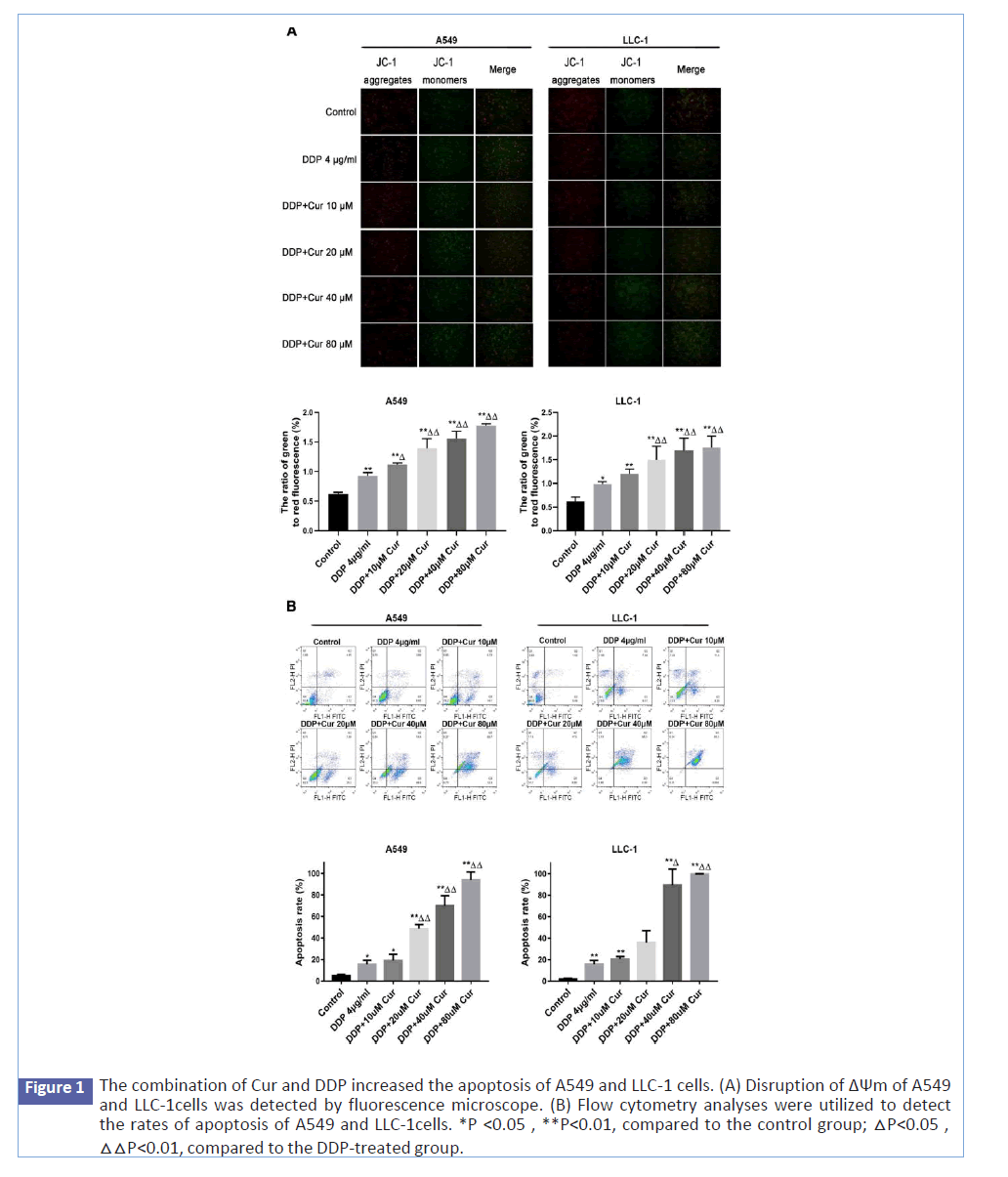
Figure 1:The combination of Cur and DDP increased the apoptosis of A549 and LLC-1 cells. (A) Disruption of ΔΨm of A549 and LLC-1cells was detected by fluorescence microscope. (B) Flow cytometry analyses were utilized to detect the rates of apoptosis of A549 and LLC-1cells. *P <0.05 , **P<0.01, compared to the control group; △P<0.05 , △△P<0.01, compared to the DDP-treated group.
Curcumin potentiated apoptosis of cisplatin in vitro by endogenous mitochondrial apoptosis pathway
Next, we investigated whether Cur increased A549 and LLC-1 cells apoptosis induced by cisplatin. MMP is an important indicator of mitochondrial function, and its loss is usually regarded as a marker of apoptosis [14,15]. The membrane-permeant JC-1 dye is widely used in researching apoptosis to assess mitochondrial condition. An increased proportion of JC-1 green/red fluorescence indicated a decrease in MMP. Compared with the control group, the ratio of JC-1 green/red fluorescence of A549 and LLC-1 cells increased in the DDP-treated group, and the combination of DDP and Cur significantly increased the ratio compared with the DDP-treated group (Figures 2A and 2B). On this basis, Annexin V-FITC/PI double staining was used to analyze the early and late apoptotic cell populations for further investigating the effects of DDP and Cur on apoptosis. The results indicated the total percentages of apoptosis increased when the A549 and LLC-1 cells were treated with DDP compared to the control group, and the combination of DDP and Cur significantly increased the total percentages of apoptosis of A549 and LLC-1 cells compared with the DDP-treated group (Figures 2C and 2D). These results demonstrated that Cur enhanced apoptosis activity induced by DDP in the endogenous mitochondrial apoptosis pathway.
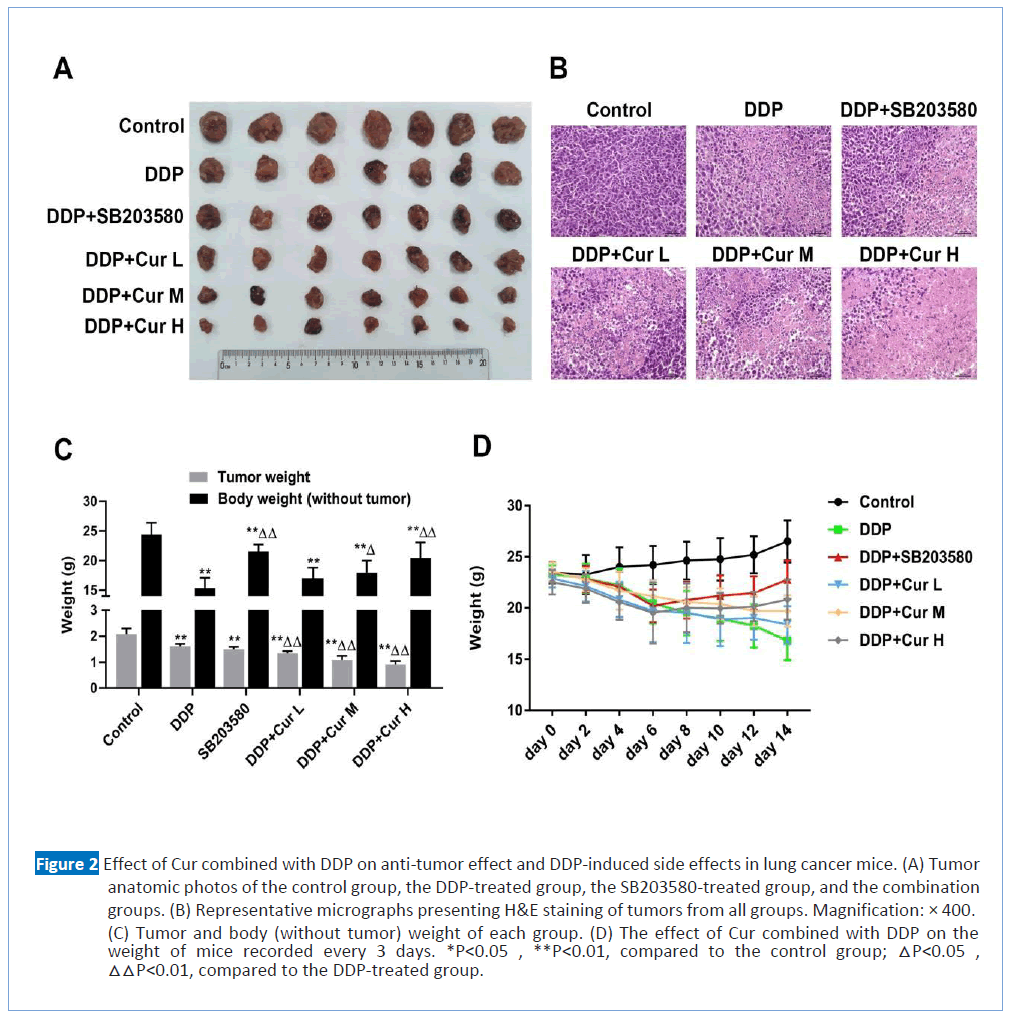
Figure 2:Effect of Cur combined with DDP on anti-tumor effect and DDP-induced side effects in lung cancer mice. (A) Tumor anatomic photos of the control group, the DDP-treated group, the SB203580-treated group, and the combination groups. (B) Representative micrographs presenting H&E staining of tumors from all groups. Magnification: × 400. (C) Tumor and body (without tumor) weight of each group. (D) The effect of Cur combined with DDP on the weight of mice recorded every 3 days. *P<0.05 , **P<0.01, compared to the control group; △P<0.05 , △△P<0.01, compared to the DDP-treated group.
Combination treatment with Cur and DDP enhances anti-tumour effects and alleviates side effect induced by DDP in LLC-1 lung cancer mice
In order to further confirm our above conclusions, we evaluated the anti-tumor effects of Cur and DDP combination therapy in vivo. As shown in Fig. 2A and C, tumor volumes were significantly reduced in the groups treated with different doses of Cur in combination with DDP, compared with the control and DDP- treated groups, especially in the high-dose Cur group. Also, we used H and E staining to assess the histologic changes in the tumor tissues and got results consistent with the above (Figure 2B). These results both revealed that the combination of DDP and Cur significantly increased the tumor suppressor effect compared with the DDP-treated group. As presented in Figures 2C and 2D, it's worth noting that the bodyweights (without tumor) of mice in all combination groups were clearly increased compared with the DDP-treated group. What’ more, we recorded the body weight of mice (with tumor) every 3 days, and the average weights of the mice in combination groups were clearly increased, compared with the DDP-treated group (Figure 3D). P38 plays an important role in DDP resistance and chemo toxicity of lung cancer [16]. In order to verify whether Cur enhanced anti-tumor effect of DDP and alleviated the chemo toxicity by inhibiting the P38 signal transduction pathway, we set up the SB203580-treated group (with DDP) as a positive control. We found that the combination of DDP and SB 203580 had a similar effect with the combination of DDP and Cur. The results indicated that Cur might promote the anti-tumor effect of DDP and reduce side effects induced by DDP through P38 inhibition. These results suggested that Cur enhanced DDP's cytotoxicity and attenuated the virulence of DDP.
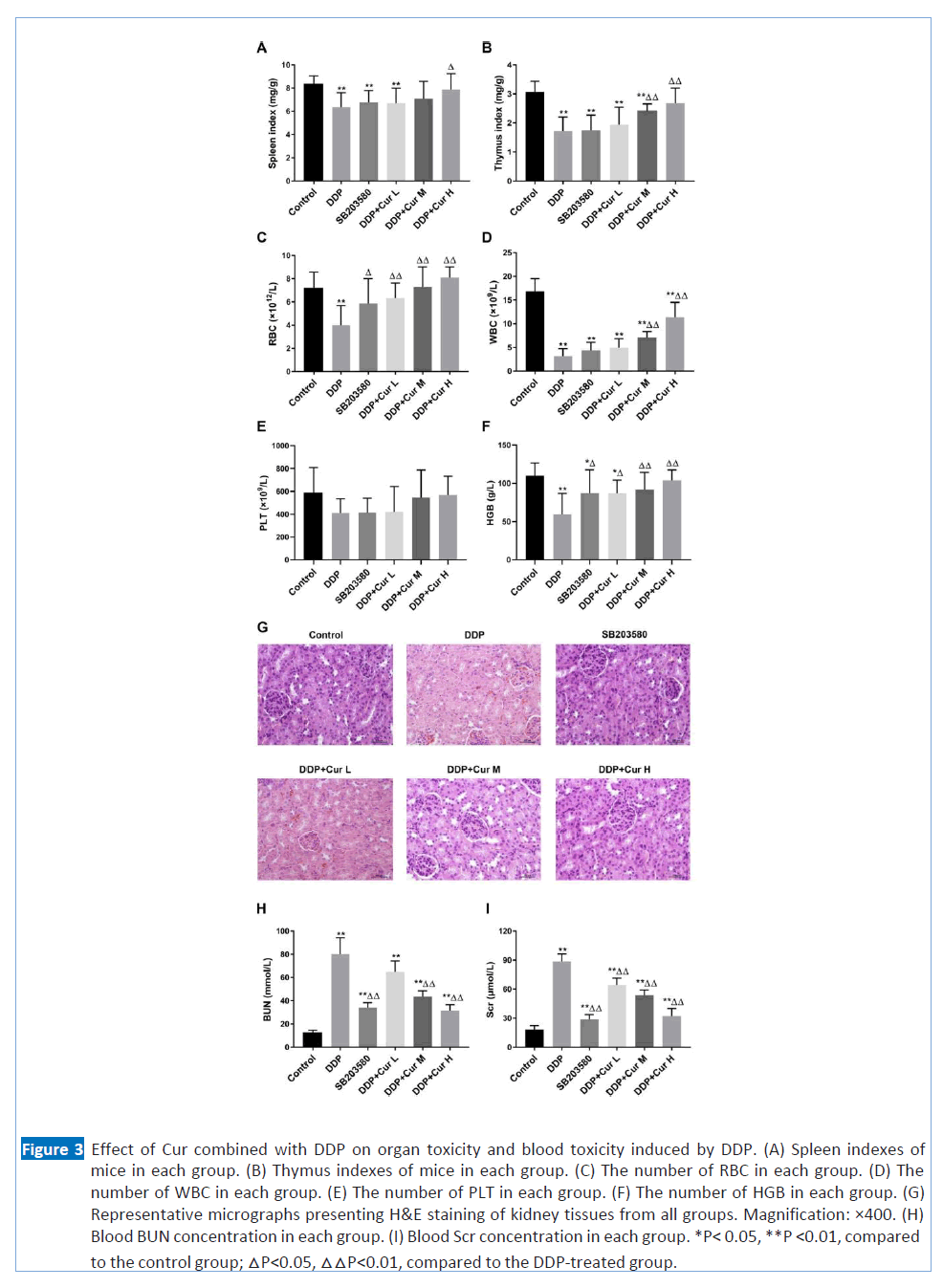
Figure 3: Effect of Cur combined with DDP on organ toxicity and blood toxicity induced by DDP. (A) Spleen indexes of mice in each group. (B) Thymus indexes of mice in each group. (C) The number of RBC in each group. (D) The number of WBC in each group. (E) The number of PLT in each group. (F) The number of HGB in each group. (G) Representative micrographs presenting H&E staining of kidney tissues from all groups. Magnification: ×400. (H) Blood BUN concentration in each group. (I) Blood Scr concentration in each group. *P< 0.05, **P <0.01, compared to the control group; △P<0.05, △△P<0.01, compared to the DDP-treated group.
Cur in combination with DDP increases immune organ indexes and relieves the blood toxicity induced by DDP
spleen and thymus indexes are regarded as important indicators of immune functions of the lung cancer bearing mice. As shown in Figures 3A and 3B, the spleen and thymus indices were significantly increased in the combined group (DDP+Cur H), compared with the DDP-treated group. In the other combined groups, these two indicators also have an upward trend, compared with the DDP-treated group. However, there was no significant difference between the SB203580-treated group and the DDP-treated group. Besides, we tested the differences in the concentrations of RBC, WBC, PLT and HGB in tumor-bearing mice to evaluate the effect of DPP combined with Cur on blood toxicity. As presented in Figures 3C, 3D and 3F, RBC, WBC, and HGB counts remarkably increased in the groups treated with different doses of Cur in combination with DDP, compared with the DDP-treated group. Even if PLT counts do not change significantly, we can still observe an upward trend in the groups treated with different doses of Cur in combination with DDP (Figure 3E). The increase of these four indicators in the SB203580-treated group compared with the DDP-treated group shows that Cur's alleviating effect of DDP-induced blood toxicity may be through inhibition of the P38 signal transduction pathway. The results suggested that Cur in combination with DDP not only has antitumor activity but protective effects on the immune and blood system.
Cur in combination with DDP alleviates nephrotoxicity induced by DDP
DDP could increase the renal oxidative stress level and damage seriously the renal histology construction and function. Therefore, we further explored the effect of Cur combined with DDP on nephrotoxicity. H&E staining is utilized to assess the histological damage of kidneys in different groups. As shown in Figure 3G, the structure of glomeruli and tubules in the control group was clear, without cell swelling and necrosis. In the DDP-treated group, the structure of kidney tissue was damaged, manifested as glomerular congestion, renal tubules, especially proximal tubule epithelial cells, turbid and swollen, nuclear fragmentation, and shedding. In the groups treated with different doses of Cur in combination with DDP and treated with SB203580, renal injury showed different degrees of reduction compared with the DPP treatment group (Figure 3G). To further validate this result, we tested the biochemical criteria of renal injury (BUN and Scr) in different groups. Compared with other groups, DDP alone remarkably increased blood Scr and BUN; in the groups treated with different doses of Cur in combination with DDP, there was a dose-dependent decrease in the above indicators; in the SB203580-treated group, these two indicators also dropped significantly (Figures 3H and 3I). The data of this study supported that Cur has protective effect against the DPP- induced nephrotoxicity and this effect may be caused by Cur's inhibition of the P38 pathway.
Cur's blocking of the oxidative stress pathway reduces DPP-induced nephrotoxicity
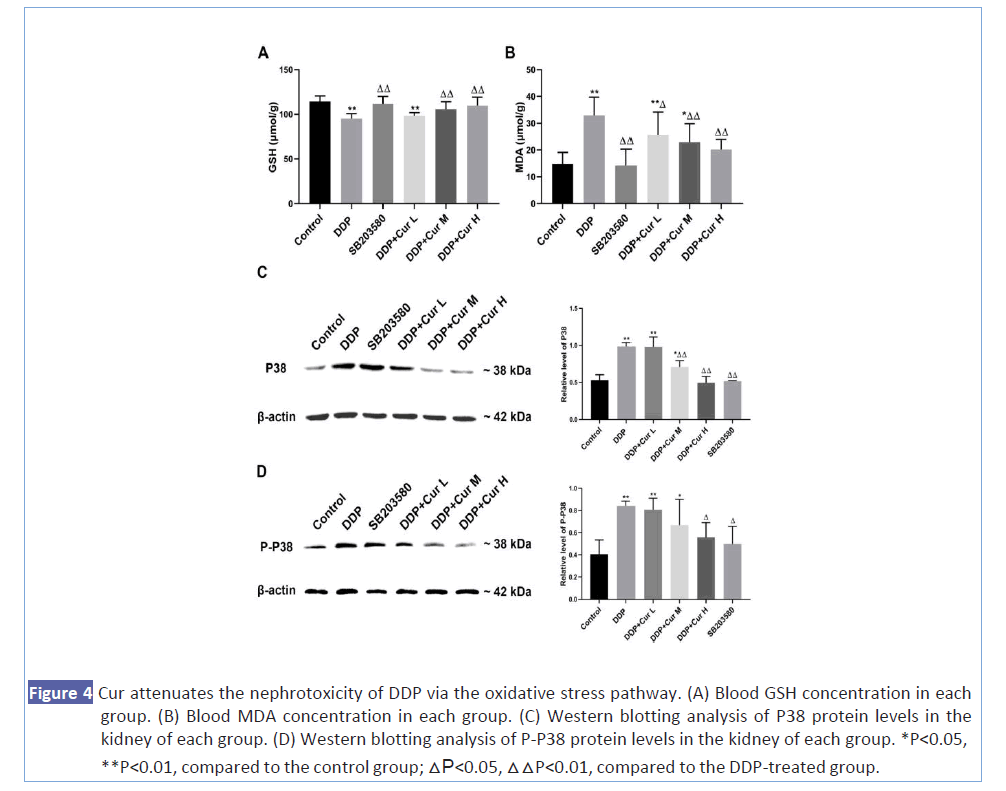
Since the increase of oxidative stress in the renal interstitium is one of the most important factors of DPP-induced nephrotoxicity, we have further explored the effect of Cur on DDP-induced oxidative stress and its mechanism. We tested the biochemical criteria of oxidative stress (GSH and MDA) in different groups. As shown in Figures 4A and 4B, we found that compared with the DDP treatment group, the GSH of mice in the groups treated with different doses of Cur in combination with DDP showed a significant dose-dependent increase, and MAD showed the opposite trend. To further confirm the correlation between the reduction of nephrotoxicity by combining Cur and DDP and the inhibition of the P38 pathway, we tested the levels of P38 and P-P38 proteins in each group. Western blotting showed that DDP alone significantly up-regulated the protein expression levels of P38 and P-P38, while the combination of Cur and DDP significantly inhibited the increase in the levels of P38 and P-P38 proteins caused by DDP, and this inhibitory effect becomes more obvious with the increase of Cur concentration (Figures 4C and 4D). Taken together, our data demonstrated that Cur effectively relieves oxidative stress by blocking the P38 signal transduction pathway to reduce DDP-induced nephrotoxicity.
Figure 4: Cur attenuates the nephrotoxicity of DDP via the oxidative stress pathway. (A) Blood GSH concentration in each group. (B) Blood MDA concentration in each group. (C) Western blotting analysis of P38 protein levels in the kidney of each group. (D) Western blotting analysis of P-P38 protein levels in the kidney of each group. *P<0.05, **P<0.01, compared to the control group; △P<0.05, △△P<0.01, compared to the DDP-treated group.
Discussion
In recent years, the morbidity and mortality of cancer patients have seriously increased. DDP is one of the most frequently used clinical chemotherapeutic agents, and its toxicity and secondary action, such as bone marrow suppression, vomiting, ototoxicity and nephrotoxicity, severely restrict its efficacy [17,18]. Therefore, the use of chemical protectants against nephrotoxicity has become a key technology [19,20]. In view of this, the main objective of our research was to survey the antitumor effect of Cur used in combination with the chemotherapy drug DDP, and to determine if Cur reduced DDP-induced renal toxicity via the P38MAPK pathway.
Many studies have shown that organ injury induced by cisplatin is mainly related to the accumulation of active oxygen and the generation of peroxidation in the tissues [21], which is primarily manifested as an increase in MDA, GSH, and activity of various antioxidant enzymes decreased [22,23]. It's worth noting that some researches have indicated that P38MAPK activated by cisplatin is the main signal transduction pathway causing toxic reactions [24]. After DNA damage, P38MAPK can be phosphorylated and activated, and it continues to activate downstream members of caspase family (such as caspase 3, 7). Activated caspase 3 can specifically lyse its substrates and DNA, and thus induce apoptosis [25]. Although study showed that curcumin enhanced anti-tumor effect of cisplatin by inhibiting P38MAPK pathway in lung cancer, there are novel findings in our study [26]. First of all, we found that after curcumin and cisplatin were given, the spleen, thymus,and kidney indexes of the mice were increased compared with cisplatin alone group. Besides, we also found that curcumin, similar to the P38MAPK kinase inhibitor, inhibited the cisplatin-activated P38MAPK signaling pathway and improved renal damage caused by cisplatin. But the way how curcumin reduced renal toxicity by cisplatin needs to be more elucidated. In the future, we will continue to demonstrate the way curcumin inhibited P38MAPK pathway to reduce DDP- induced renal toxicity.
Overall, our study demonstrated that curcumin combined with cisplatin increased anti-tumor effect in lung cancer and induced apoptosis. Curcumin can also reduce to DDP-induced renal toxicity by P38MAPK pathway. Further study needs to be conducted to investigate the way curcumin reduced DDP-induced nephrotoxicity. Our research still provides treatment strategies for patients with lung cancer to reduce renal toxicity caused by DDP.
Conclusion
In conclusion, our investigation suggested that the combination curcumin and cisplatin had synergistic effects against lung cancer in vitro through mitochondrial apoptosis pathway. Reduction of nephrotoxicity induced by cisplatin through the oxidative stress pathway was observed in vivo. Therefore, we believed that curcumin has the potential to become a more effective strategy for combination therapy to reduce renal toxicity of lung cancer patients.
Acknowledgment
This work was supported by the Jinzhou Medical University.
Statement of Ethics
Animal experiments have been approved by the Animal Ethics Committee of Jinzhou Medical University. The permit number is SYXK(Liaoning)2014-0010.
Conflicts of interest statement
The authors have no conflicts of interest to declare.
Funding Sources
The fund project of this paper is the science and technology innovation talent project of Liaoning Province. It is the Education Department of Liaoning Province. The project number is LR2019028
Author Contributions
All the authors participated in writing and giving feedback on the manuscript. All authors have read and approved the final manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for publication
Not applicable.
References
- Izdebska M, Zielińska W, Hałas-Wiśniewska M, Mikołajczyk K (2019) The cytotoxic effect of oxymatrine on basic cellular processes of A549 non-small lung cancer cells. Acta Histochem 121: 724-731.
- Izdebska M, Zielińska W, Hałas-Wiśniewska M, Mikołajczyk K (2019) The cytotoxic effect of oxymatrine on basic cellular processes of A549 non-small lung cancer cells. Acta Histochem 121: 724-731.
- Wakelee H, Kelly K, Edelman MJ (2014) 50 Years of progress in the systemic therapy of non-small cell lung cancer. Am Soc Clin Oncol Educ Book 34: 177-189.
- Zhou GZ, Li AF, Sun YH, Sun GC (2018) A novel synthetic curcumin derivative MHMM-41 induces ROS-mediated apoptosis and migration blocking of human lung cancer cells A549. Biomed Pharmacother. 103: 391-398.
- Huang YC, Tsai MS, Hsieh PC, Shih JH, Wang TS, et al. (2017) Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol Appl Pharmacol 329: 128-139.
- Hazra B, Ghosh S, Kumar A, Pandey BN (2011) The prospective role of plant products in radiotherapy of cancer: a current overview. Front Pharmacol 2: 94.
- Campbell NP, Kindler HL (2011) Update on malignant pleural mesothelioma. Semin Respir Crit Care Med 32: 102-110.
- Khan AR, Farooqui MO (2019) Curcumin: An unconventional treatment - letter to the editor. J Pak Med Assoc 69: 924.
- Zhou GZ, Li AF, Sun YH, Sun GC (2018) A novel synthetic curcumin derivative MHMM-41 induces ROS-mediated apoptosis and migration blocking of human lung cancer cells A549. Biomed Pharmacother. 103: 391-398.
- Schaeppi U, Heyman IA, Fleischman RW, Rosenkrantz H, Ilievski V, et al. (1973) cis-Dichlorodiammineplatinum(II) (NSC-119 875): preclinical toxicologic evaluation of intravenous injection in dogs, monkeys and mice. Toxicol Appl Pharmacol 25: 230-241.
- Huang YC, Tsai MS, Hsieh PC, Shih JH, Wang TS, et al. (2017) Galangin ameliorates cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammation and cell death in mice through inhibition of ERK and NF-kappaB signaling. Toxicol Appl Pharmacol 329: 128-139.
- . Goffin J, Lacchetti C, Ellis PM, Ung YC, Evans WK, (2010) Lung Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-Based Care. First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol 5: 260-274.
- Campbell NP, Kindler HL (2011) Update on malignant pleural mesothelioma. Semin Respir Crit Care Med 32: 102-110.
- Rafiee Z, Nejatian M, Daeihamed M, Jafari SM (2019) Application of different nano carriers for encapsulation of curcumin. Crit Rev Food Sci Nutr 59: 3468-3497.
- Cetin R, Devrim E, Kiliçoğlu B, Avci A, Candir O, et al. (2006) Cisplatin impairs antioxidant system and causes oxidation in rat kidney tissues: possible protective roles of natural antioxidant foods. J Appl Toxicol 26: 42-46.
- Schaeppi U, Heyman IA, Fleischman RW, Rosenkrantz H, Ilievski V, et al. (1973) cis-Dichlorodiammineplatinum(II) (NSC-119 875): preclinical toxicologic evaluation of intravenous injection in dogs, monkeys and mice. Toxicol Appl Pharmacol 25: 230-241.
- Gonzales-Vitale JC, Hayes DM, Cvitkovic E, Sternberg SS (1977) The renal pathology in clinical trials of cis-platinum (II) diamminedichloride. Cancer 39: 1362-1371.
- Di Desidero T, Antonelli A, Orlandi P, Ferrari SM, Fioravanti A, et al. (2017) Synergistic efficacy of irinotecan and sunitinib combination in preclinical models of anaplastic thyroid cancer. Cancer Lett 411:3 5-43.
- Cui H, Li T, Wang L, Su Y, Xian CJ (2016) Dioscorea bulbifera polysaccharide and cyclophosphamide combination enhances anti-cervical cancer effect and attenuates immunosuppression and oxidative stress in mice. Sci Rep 5:19185.
- Cullen KJ, Yang Z, Schumaker L, Guo Z (2007) Mitochondria as a critical target of the chemotheraputic agent cisplatin in head and neck cancer. J Bioenerg Biomembr 39: 43-50.
- . He L, Zhang Y, Kang N, Wang Y, Zhang Z, et al. (2019) Anemoside B4 attenuates nephrotoxicity of cisplatin without reducing anti-tumor activity of cisplatin. Phytomedicine 56: 136-146.
- Sánchez Rodríguez MT, Collado Vázquez S, Martín Casas P, Cano de la Cuerda R (2018) Neurorehabilitation and apps: A systematic review of mobile applications. Neurologia33: 313-326.
- Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, et al. (2000) Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol 278: F726-36.
- Khan R, Khan AQ, Qamar W, Lateef A, Tahir M, et al. (2012) Chrysin protects against cisplatin-induced colon. toxicity via amelioration of oxidative stress and apoptosis: probable role of p38MAPK and p53. Toxicol Appl Pharmacol 258: 315-329.
- Cummings BS, Schnellmann RG (2002) Cisplatin-induced renal cell apoptosis: caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther 302: 8-17.
- Tung CL, Jian YJ, Chen JC, Wang TJ, Chen WC, et al. (2016) Curcumin downregulates p38 MAPK-dependent X-ray repair cross-complement group 1 (XRCC1) expression to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Naunyn Schmiedebergs Arch Pharmacol 389: 657-666.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences