Blocking Fructose could be a Novel Approach against Cancer
Takahiko Nakagawa, Miguel A. Lanaspa, Ana Andres-Hernando, Hideto Kojima and Richard J. Johnson
Takahiko Nakagawa1,2*, Miguel A. Lanaspa3, Ana Andres-Hernando3, Hideto Kojima2 and Richard J. Johnson3
1Department of Nephrology, Rakuwakai Otowa Hospital, Kyoto, Japan
2Department of Biochemistry, Shiga University of Medical Science, Otsu, Japan
3Division of Renal Diseases and Hypertension, University of Colorado Denver, Aurora, Colorado, USA
- Corresponding Author:
- Dr. Takahiko Nakagawa
Department of Nephrology, Rakuwakai Otowa Hospital 2 Otowa-Chinji-cho, Yamashina-ku, Kyoto, Japan
Tel: (+81) 81-75-593-4111
E-mail: nakagawt@gmail.com
Received date: November 24, 2020; Accepted date: December 08, 2020; Published date: December 15, 2020
Citation: Nakagawa T, Lanaspa MA, Andres-Hernando A, Kojima H, Johnson RJ (2020) Blocking Fructose could be a Novel Approach against Cancer. J Clin Exp Nephrol Vol.5 No.5: 93.
Abstract
Despite of the appreciation for the recent advance of anti-cancer therapy, the effect remains unsatisfactory. Since obesity and metabolic syndrome are strongly associated with cancers, there might be a common pathway underlying these disorders. Recently, sugar, particularly fructose, has emerged as a potential driving force to develop obesity and metabolic syndrome, and also associated with several types of cancers, suggesting that fructose might be a link between these disorders as fructose, as opposed to glucose, drives several pathways for cancer growth. A unique property of fructose is to produce uric acid which causes an imbalance favoring glycolysis over mitochondrial respiration, resembling the Warburg effect in cancer cells. Fructose also activates the pentose phosphate pathway, resulting in the synthesis of nucleotide and amino acid. Lactate production is also accelerated as an alternative energy while de novo lipogenesis links to energy supply and membrane formation for proliferating cells. Here, we discuss the role of fructose in several types of cancers and propose that blocking fructose metabolism could be an additional therapy to alleviate the cancer growth.
Keywords
Uric acid; Lactate; Glycolysis; Mitochondria; The warburg effect; Pentose phosphate pathway; Lipogenesis
Introduction
Metabolic syndrome is associated with cancer. Cohort studies showed that the presence of metabolic syndrome was associated with liver, colorectal, and bladder cancer in men, and with endometrial, pancreatic, breast postmenopausal, rectal, and colorectal cancers [1]. There might be a common mechanism driving these disorders. While several mechanisms are now proposed, unhealthy diet could contribute to an increase in cancer risk [2,3].
Glucose has been thought to be a major energy source for cancer growth [4-7]. In 1924 Otto Warburg initially mentioned that cancer cells, as opposed to normal cells, exhibit a unique property to ferment glucose into lactate even in the presence of sufficient oxygen [8,9]. Importantly, the Warburg effect is characterized by a low respiration rate in mitochondria despite of relatively the high rate of glucose uptake [10]. However, the fact is that some types of cancers fail to utilize glucose as an energy source [11], suggesting that there might be another source of energy for cancer growth.
Fructose is a simple sugar in fruits that has a role in storing fat and glycogen, developing insulin resistance, and increasing sodium reabsorption, all of which are likely survival processes for wild animals, great apes, and humans during time of food shortage [12]. However, we are currently consuming a large amount of fructose in the form of sugar because sugar makes foods tasty. As a result, fructose has emerged as culprits for the current epidemic of obesity, diabetes and metabolic syndrome in the modern society [13]. The rise in fructose consumption over the last century has paralleled the rising prevalence of obesity and metabolic syndrome as well as some types of cancers, leading to the hypothesis that the excessive amount of fructose could be a link between these disorders.
Pathways of fructose-driven cancer growth
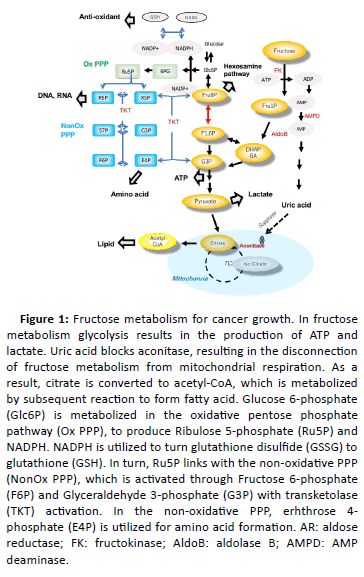
Several factors are required for cancer growth, including energy source, nucleotide, lipid, and redox balance. The Warburg effect is also important. Fructose is metabolized through four pathways and contributes to meet the demand for cancer (Figure 1).
Figure 1: Fructose metabolism for cancer growth. In fructose metabolism glycolysis results in the production of ATP and lactate. Uric acid blocks aconitase, resulting in the disconnection of fructose metabolism from mitochondrial respiration. As a result, citrate is converted to acetyl-CoA, which is metabolized by subsequent reaction to form fatty acid. Glucose 6-phosphate (Glc6P) is metabolized in the oxidative pentose phosphate pathway (Ox PPP), to produce Ribulose 5-phosphate (Ru5P) and NADPH. NADPH is utilized to turn glutathione disulfide (GSSG) to glutathione (GSH). In turn, Ru5P links with the non-oxidative PPP (NonOx PPP), which is activated through Fructose 6-phosphate (F6P) and Glyceraldehyde 3-phosphate (G3P) with transketolase (TKT) activation. In the non-oxidative PPP, erhthrose 4- phosphate (E4P) is utilized for amino acid formation. AR: aldose reductase; FK: fructokinase; AldoB: aldolase B; AMPD: AMP deaminase.
Glycolysis for energy production
The first enzyme for fructose metabolism is fructokinase (known as ketohexokinase; KHK), which phosphorylates fructose to produce Fructose 1-phosphate (Fru1P). Fructokinase is predominantly expressed in the liver, the kidney and other organs, but the liver is the primary site for dietary fructose metabolism [14,15]. Interestingly, recent studies have shown that microbiota in the gastrointestinal tract plays a substantial role in fructose metabolism [16,17]. Furthermore, recent experiments using mice with the selective knockout of fructokinase in the liver or intestine document that, while the intestine has an important role in clearance and intake, the liver metabolism of fructose is responsible for most of the features of metabolic syndrome [18]. Fru1P is subsequently metabolized by aldolase B and triokinase to dihydroxyacetone phosphate and glyceraldehyde-3-phosphate to enter the glycolytic pathway distal to phosphofructokinase. Recently, a key role of aldolase B in cancer growth was shown that aldolase B mediates colon cancer liver metastasis and that reducing dietary fructose diminishes liver metastatic growth in mice [19].
Subsequently, glyceraldehyde-3-phosphate is metabolized into pyruvate in glycolytic pathway to produce ATP and NADH (Figure 2). Pyruvate is further converted into lactate using lactate dehydrogenase. This reaction is usually stimulated by low oxygen, but is accelerated by fructose even under aerobic condition [20]. The lactate seems to be an energy for cancer growth as blocking lactate production by blocking LDH-A with a chemical inhibitor or gene deletion ameliorated angiogenesis and inhibited cancer cell proliferation [21,22].
In addition, fructose may facilitate glucose utilization by activating glucokinase. Glucokinase (hexokinase (HK) IV) is negatively regulated by glucokinase regulator protein (GKRP) binding with Fru6P and is sequestered in the nucleus, but in the presence of fructose Fru6P is replaced by Fru1P to release glucokinase from GKRP into cytosol for stimulating glucose uptake and glycolysis [23-25]. However, glucokinase is usually expressed only in hepatocyte and pancreatic β cells, but is less expressed in cancer cells [26] so that the role of glucokinase needs to be clarified in the cancer. A recently discovered enzyme, ADP-dependent glucokinase (ADPGK) could be involved in the cancer growth [27] as this enzyme is highly expressed in human tumors and tumor cell lines [28]. ADPGK may function under stress conditions where the supply of ATP is limited [29]. In turn, it has been well documented that HK II is overexpressed in cancer cells to facilitate glycolysis, suppress the death of cancer cells, and promote metastasis [26].
Pentose phosphate pathway for nucleotide, amino acid and redo balance
The pentose phosphate pathway (PPP) is activated by fructose, and is composed of two sections, oxidative pathway and non-oxidative pathway (Figure 2). Glucose 6-phosphate, a fructose metabolite, is metabolized by three sequential reactions to produce NADPH for reducing oxidative stress with GSH production in the oxidative pathway. In turn, two forms of fructose carbon backbones, fructose 6-phosphate and glyceraldehyde-3-phosphate, are catalyzed by transketolase to enter the non-oxidative pathway. The activation of the transketolase drives nucleotide formation through ribose 5- phosphate while erythrose 4-phosphate are metabolized into amino acid. Liu et al. found that using pancreatic cancer cells, fructose and glucose exhibited the same effect on cell proliferation, but their intracellular metabolism was different [30]. The productions of lactate, CO2, and fatty acid were significantly higher in cells with glucose compared to those with fructose. However, fructose was more potent to activate the non-oxidative pentose phosphate pathway in association with intracellular transketolase activation, and accelerate ribose synthesis and uric acid production [30]. Alternatively, activated hexokinase could convert fructose into fructose 6-phosphate, which might be at link between glycolysis and the non-oxidative PPP in cancer cells.
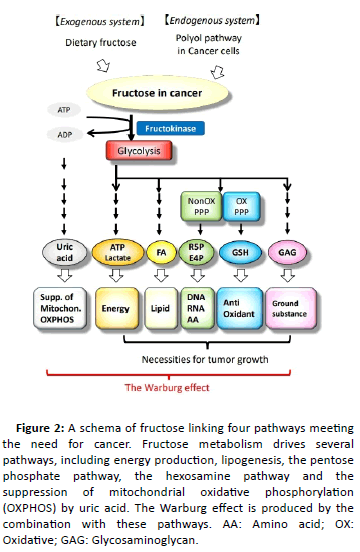
Figure 2: A schema of fructose linking four pathways meeting the need for cancer. Fructose metabolism drives several pathways, including energy production, lipogenesis, the pentose phosphate pathway, the hexosamine pathway and the suppression of mitochondrial oxidative phosphorylation (OXPHOS) by uric acid. The Warburg effect is produced by the combination with these pathways. AA: Amino acid; OX:
Oxidative; GAG: Glycosaminoglycan.
Lipogenesis
Lipids are likely required as an energy source and for membrane formation, and as signaling molecules in cancer (Figure 2). Therefore, cancer cells actively uptake lipid and promote de novo lipogenesis [31,32]. Fructose is metabolized in glycolytic pathway to provide the carbon backbone as acetyl- CoA for de novo lipogenesis, and also promotes fatty acid synthesis to form palmitate. In turn, glyceraldehyde-3- phosphate carrying fructose carbon backbone is also utilized to form triglyceride. Importantly, triglyceride levels were elevated in several types of cancer and associated with an increased risk of cancer [33-35]. Fructose acts as a carbon source and stimulates some intracellular signaling, including Carbohydrateresponsive element-binding protein (ChREBP) [36,37] and glucokinase regulatory protein (GKRP) [23,24]. In turn, a recent study using a mouse model demonstrated that fructosemediated fatty liver disease is likely mediated by impairment of fatty acid oxidation due to deacetylation of Acyl-CoA dehydrogenase, long chain (ACADL) and carnitine palmitoyltransferase 1α (CPT1α) [38].
Uric Acid for W arbug effect
Fructokinase activation rapidly sequesters a phosphate, consequently activating AMP deaminase to cleave AMP to IMP [39]. However, the phosphate levels subsequently increase due to the slower aldolase reaction with Fru1P. This reaction is further accentuated by the increased IMP, which is an aldolase B inhibitor [40]. Sequential enzymatic activation metabolizes IMP and eventually produce uric acid [13,37,41]. Recently, our research group has attempted to clarify the role of uric acid in fructose metabolism [13,41]. We found that uric acid could prevent fructose metabolites from channeling into mitochondrial oxidation in the human hepatocellular carcinoma cell line HepG2 [42]. A potential mechanism was the ability of uric acid to suppress aconitase activity in the mitochondria, and to disconnect fructose metabolites from mitochondrial oxidation. Since aconitase lies at the junction of acetyl-CoA oxidation, blocking aconitase leads to acetyl-CoA shuttling out of the mitochondria, resulting in the accumulation of citrate in the cytosol, where citrate was utilized for lipid synthesis by sequential ATP-citrate lyase and fatty-acid synthase [42]. A key point is that uric acid does not totally block aconitase activity so that mitochondria respiration remains operated (Figure 2).
In terms of the Warburg effect, several investigators have reevaluated the role of mitochondria, and showed that mitochondria were commonly required for tumor growth. A key point is that the glycolytic rate may far exceed the maximal rate of mitochondrial pyruvate oxidation, thus making lactate excretion unavoidable in the presence of abundant glucose [10]. In fact, Weinberg et al indicated that tumor cells would require mitochondria-derived reactive oxygen species (ROS), but not OXPHOS, for cell proliferation [43].
Role of fructose in Cancer
The Nurses’ Health Study showed that fructose intake was the strongest risk factor for pancreatic cancer in subjects who were overweight or sedentary [44]. Subsequently, the combined analysis with Nurses’ Health Study and the Health Professionals Follow-up Study showed that sugar-sweetened beverage consumption was associated with an increase in risk for pancreatic cancer among women, but not men [45]. Alternatively, a food-frequency questionnaire with 77,797 women and men in Sweden also found that high consumption of sugar and high-sugar foods resulted in a greater risk of pancreatic cancer [46]. In addition, serum concentration of fructose was also higher in patients with pancreatic cancer than healthy patients [47].
Several clinical studies found a positive association between sugar/fructose intake and the risk of several types of cancers, including colorectal cancer [48] and breast cancer [49]. For breast cancer cells, fructose caused greater adhesion of cancer cells to endothelium and enhanced aggressive migration compared to glucose [50]. Jiang et al. also found that a fructose diet stimulated tumor growth of breast cancer with the expression of 12-lipoxygenase and the production of the arachidonate metabolite 12-hydoroxy-5Z,8Z,10E,14Z-eicosatetraenoic acid production, and the spread of metastatic tumors in the lung, compared to either a glucose or control starch diet [51].
Other types of cancers would be also promoted by fructose. Weng et al. showed that compared to glucose, fructose was more potent to produce ATP and fatty acids for lung cancers [52]. The causal role of fructose could be also supported for by the fact an increase in GLUT5 expression was associated with poor prognosis in patients with lung adenocarcinoma [52]. Likewise, AML patients were found to exhibited an increase in GLUT5 gene expression in myeloid cells, while increased fructose utilization was associated with poor clinical outcomes [53]. In brain, it was also found that fructokinase and GLUT5 were highly expressed in glioma and were also correlated with malignancy and poor survival of glioma patients [54,55] (Table 1).
| Type of cancer | References |
|---|---|
| Pancreatic cancer | [44] [45] [46] [47] [60] [30] |
| Colorectal cancer | [48] [56] |
| Breast cancer | [49] [50] [51] |
| Lung cancer | [52] |
| Acute myelogenous leukemia | [53] |
| Glioma | [54,55] |
| Hepatocellular carcinoma | [61] |
Table 1: Types of cancers in which fructose contributes to tumor growth.
Blocking fructose metabolism in cancer
Goncalves et al. investigated the effects of dietary fructose (~3% of total daily caloric intake) in adenomatous polyposis coli mutant mice, which are predisposed to develop intestinal tumors [56]. The fructose-treated mice showed a substantial increase in tumor size and tumor grade in the absence of obesity and metabolic syndrome. They confirmed that fructose was converted to fructose-1-phosphate, leading to activation of glycolysis and increased synthesis of fatty acids that support tumor growth in tumor, and that knocking down fructose metabolism by deleting fructokinase (ketohexokinase) gene suppressed cancer growth in response to HFCS [56].
Alternatively, an inhibiting aldolase B could also block fructose metabolism, but the anti-cancerous effect remains controversial. Several studies showed that fructose-mediated aldolase B drives either colon cancer [57,58] or its liver metastasis [19]. In turn, a recent study has shown that systemic or liver-specific aldolase B knockout promotes tumorigenesis in mice through enhancing G6PD activity and pentose phosphate pathway metabolism [59-61].
Conclusion
An increased fructose intake is associated with a risk for several types of cancer, suggesting that fructose might be an alternative fuel for cancer growth. A mechanism could be accounted for by the ability of fructose to drive four pathways for cancer growth. Necessities for cancer growth including energy, nucleic acid, amino acid, redox balance and the Warburg effects could be provided by fructose metabolism. Blocking fructose metabolism cannot eliminate cancer, but may slow the progression of cancer growth.
Acknowledgements
Not applicable
References
- Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D (2012) Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care 35: 2402-2411.
- Esposito K, Ciardiello F, Giugliano D (2014) Unhealthy diets: A common soil for the association of metabolic syndrome and cancer. Endocrine 46: 39-42.
- Cowey S, Hardy RW (2006) The metabolic syndrome: A high-risk state for cancer? Am J Pathol 169: 1505-1522.
- Hosios AM, Hecht VC, Danai LV, Johnson MO, Rathmell JC, et al. (2016) Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev Cell 36: 540-549.
- Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, et al. (2011) Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet 43: 869-874.
- Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, et al. (2012) Oncogenic kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149: 656-670.
- DeBerardinis RJ, Chandel NS (2020) We need to talk about the Warburg effect. Nat Metab 2: 127-129.
- Warburg O (1956) On respiratory impairment in cancer cells. Science 124: 269-270.
- Warburg O (1956) On the origin of cancer cells. Science 123: 309-314.
- DeBerardinis RJ, Chandel NS (2020) We need to talk about the Warburg effect. Nat Metab 2: 127-129.
- Lassen U, Daugaard G, Eigtved A, Damgaard K, Friberg L (1999) 18F-FDG whole body positron emission tomography (PET) in patients with unknown primary tumours (UPT). Eur J Cancer 35: 1076-1082.
- Johnson RJ, Stenvinkel P, Andrews P, Sanchez-Lozada LG, Nakagawa T, et al. (2019) Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts. J Intern Med.
- Nakagawa T, Tuttle KR, Short RA, Johnson RJ (2005) Hypothesis: Fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol 1: 80-86.
- Diggle CP, Shires M, Leitch D, Brooke D, Carr IM, et al. (2009) Ketohexokinase: Expression and localization of the principal fructose-metabolizing enzyme. J Histochem Cytochem 57: 763-774.
- Ishimoto T, Lanaspa MA, Le MT, Garcia GE, Diggle CP, et al. (2012) Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc Natl Acad Sci USA 109: 4320-4325.
- Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, et al. (2018) The small intestine converts dietary fructose into glucose and organic acids. Cell Metab 27: 351-361.
- Zhao S, Jang C, Liu J, Uehara K, Gilbert M, et al. (2020) Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature 579: 586-591.
- Andres-Hernando A, Orlicky DJ, Kuwabara M, Ishimoto T, Nakagawa T, et al. (2020) Deletion of fructokinase in the liver or in the intestine reveals differential effects on sugar-induced metabolic dysfunction. Cell Metab in press.
- Bu P, Chen KY, Xiang K, Johnson C, Crown SB, et al. (2018) Aldolase B-Mediated Fructose Metabolism Drives Metabolic Reprogramming of Colon Cancer Liver Metastasis. Cell Metab 27: 1249-1262.
- Lecoultre V, Benoit R, Carrel G, Schutz Y, Millet GP, et al. (2010) Fructose and glucose co-ingestion during prolonged exercise increases lactate and glucose fluxes and oxidation compared with an equimolar intake of glucose. Am J Clin Nutr 92: 1071-1079.
- San-Millan I, Brooks GA (2017) Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 38: 119-133.
- Vegran F, Boidot R, Michiels C, Sonveaux P, Feron O (2011) Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-kappaB/IL-8 pathway that drives tumor angiogenesis. Cancer Res 71: 2550-2560.
- Brown KS, Kalinowski SS, Megill JR, Durham SK, Mookhtiar KA (1997) Glucokinase regulatory protein may interact with glucokinase in the hepatocyte nucleus. Diabetes 46: 179-186.
- Niculescu L, Veiga-da-Cunha M, Van Schaftingen E (1997) Investigation on the mechanism by which fructose, hexitols and other compounds regulate the translocation of glucokinase in rat hepatocytes. Biochem J 321: 239-246.
- Lanaspa MA, Andres-Hernando A, Orlicky DJ, Cicerchi C, Jang C, et al. (2018) Ketohexokinase C blockade ameliorates fructose-induced metabolic dysfunction in fructose-sensitive mice. J Clin Invest 128: 2226-2238.
- Mathupala SP, Ko YH, Pedersen PL (2006) Hexokinase II: Cancer's double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene 25: 4777-4786.
- Richter S, Morrison S, Connor T, Su J, Print CG, et al. (2013) Zinc finger nuclease mediated knockout of ADP-dependent glucokinase in cancer cell lines: Effects on cell survival and mitochondrial oxidative metabolism. PLoS One 8: e65267.
- Richter S, Richter JP, Mehta SY, Gribble AM, Sutherland-Smith AJ, et al. (2012) Expression and role in glycolysis of human ADP-dependent glucokinase. Mol Cell Biochem 364: 131-145.
- Ronimus RS, Morgan HW (2004) Cloning and biochemical characterization of a novel mouse ADP-dependent glucokinase. Biochem Biophys Res Commun 315: 652-658.
- Liu H, Huang D, McArthur DL, Boros LG, Nissen N, et al. (2010) Fructose induces transketolase flux to promote pancreatic cancer growth. Cancer Res 70: 6368-6376.
- Snaebjornsson MT, Janaki-Raman S, Schulze A (2020) Greasing the wheels of the cancer machine: The role of lipid metabolism in cancer. Cell Metab 31: 62-76.
- Daniels VW, Smans K, Royaux I, Chypre M, Swinnen JV, et al. (2014) Cancer cells differentially activate and thrive on de novo lipid synthesis pathways in a low-lipid environment. PLoS One 9: e106913.
- Potischman N, McCulloch CE, Byers T, Houghton L, Nemoto T, et al. (1991) Associations between breast cancer, plasma triglycerides, and cholesterol. Nutr Cancer 15: 205-215.
- Ulmer H, Borena W, Rapp K, Klenk J, Strasak A, et al. (2009) Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br J Cancer 101: 1202-1206.
- Siemianowicz K, Gminski J, Stajszczyk M, Wojakowski W, Goss M, et al. (2000) Serum total cholesterol and triglycerides levels in patients with lung cancer. Int J Mol Med 5: 201-205.
- Lee HJ, Cha JY (2018) Recent insights into the role of ChREBP in intestinal fructose absorption and metabolism. BMB Rep 51: 429-436.
- Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, Li N, Roncal-Jimenez CA, et al. (2012) Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One 7: e47948.
- Softic S, Meyer JG, Wang GX, Gupta MK, Batista TM, et al. (2019) Dietary sugars alter hepatic fatty acid oxidation via transcriptional and post-translational modifications of mitochondrial proteins. Cell Metab 30: 735-753.
- Maenpaa PH, Raivio KO, Kekomaki MP (1968) Liver adenine nucleotides: Fructose-induced depletion and its effect on protein synthesis. Science 161: 1253-1254.
- Woods HF, Eggleston LV, Krebs HA (1970) The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J 119: 501-510.
- Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, et al. (2006) A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 290: F625-631.
- Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, et al. (2012) Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and independent fatty liver. J Biol Chem 287: 40732-40744.
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, et al. (2010) Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci USA 107: 8788-8793.
- Michaud DS, Liu S, Giovannucci E, Willett WC, Colditz GA, et al. (2002) Dietary sugar, glycemic load, and pancreatic cancer risk in a prospective study. J Natl Cancer Inst 94: 1293-1300.
- Schernhammer ES, Hu FB, Giovannucci E, Michaud DS, Colditz GA, et al. (2005) Sugar-sweetened soft drink consumption and risk of pancreatic cancer in two prospective cohorts. Cancer Epidemiol Biomarkers Prev 14: 2098-2105.
- Larsson SC, Bergkvist L, Wolk A (2006) Consumption of sugar and sugar-sweetened foods and the risk of pancreatic cancer in a prospective study. Am J Clin Nutr 84: 1171-1176.
- Hui H, Huang D, McArthur D, Nissen N, Boros LG, et al. (2009) Direct spectrophotometric determination of serum fructose in pancreatic cancer patients. Pancreas 38: 706-712.
- Michaud DS, Fuchs CS, Liu S, Willett WC, Colditz GA, et al. (2005) Dietary glycemic load, carbohydrate, sugar, and colorectal cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 14: 138-147.
- Terry PD, Jain M, Miller AB, Howe GR, Rohan TE (2003) Glycemic load, carbohydrate intake, and risk of colorectal cancer in women: A prospective cohort study. J Natl Cancer Inst 95: 914-916.
- Monzavi-Karbassi B, Hine RJ, Stanley JS, Ramani VP, Carcel-Trullols J, et al. (2010) Fructose as a carbon source induces an aggressive phenotype in MDA-MB-468 breast tumor cells. Int J Oncol 37: 615-622.
- Jiang Y, Pan Y, Rhea PR, Tan L, Gagea M, et al. (2016) A sucrose-enriched diet promotes tumorigenesis in mammary gland in part through the 12-lipoxygenase pathway. Cancer Res 76: 24-29.
- Weng Y, Zhu J, Chen Z, Fu J, Zhang F (2018) Fructose fuels lung adenocarcinoma through GLUT5. Cell Death Dis 9: 557.
- Chen WL, Wang YY, Zhao A, Xia L, Xie G, et al. (2016) Enhanced fructose utilization mediated by SLC2A5 is a unique metabolic feature of acute myeloid leukemia with therapeutic potential. Cancer Cell 30: 779-791.
- Gao W, Li N, Li Z, Xu J, Su C (2018) Ketohexokinase is involved in fructose utilization and promotes tumor progression in glioma. Biochem Biophys Res Commun 503: 1298-1306.
- Su C, Li H, Gao W (2018) GLUT5 increases fructose utilization and promotes tumor progression in glioma. Biochem Biophys Res Commun 500: 462-469.
- Goncalves MD, Lu C, Tutnauer J, Hartman TE, Hwang SK, et al. (2019) High-fructose corn syrup enhances intestinal tumor growth in mice. Science 363: 1345-1349.
- Li Q, Li Y, Xu J, Wang S, Xu Y, et al. (2017) Aldolase B overexpression is associated with poor prognosis and promotes tumor progression by epithelial-mesenchymal transition in colorectal adenocarcinoma. Cell Physiol Biochem 42: 397-406.
- Lian J, Xia L, Chen Y, Zheng J, Ma K, et al. (2019) Aldolase B impairs DNA mismatch repair and induces apoptosis in colon adenocarcinoma. Pathol Res Pract 215: 152597.
- Li M, He X, Guo W, Yu H, Zhang S, et al. (2020) Aldolase B suppresses hepatocellular carcinogenesis by inhibiting G6PD and pentose phosphate pathways. Nat Cancer 1: 735-747.
- Gatenby RA, Gillies RJ (2004) Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4: 891-899.
- Li X, Qian X, Peng LX, Jiang Y, Hawke DH, et al. (2016) A splicing switch from ketohexokinase-C to ketohexokinase-A drives hepatocellular carcinoma formation. Nat Cell Biol 18: 561-571.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences