Autosomal Recessive Trait of UMOD Gene in Consanguineous Family Presented with End Stage Renal Disease
Abdulmecit Yildiz, Orhan Gorukmez, Aysegul Oruc, Cuma Bulent Gul, Suat Akgur, Oktay Unsal, M Fethullah Aydin and Alparslan Ersoy
DOI10.21767/2472-5056.100072
Abdulmecit Yildiz1*, Orhan Gorukmez2, Aysegul Oruc1, Cuma Bulent Gul3, Suat Akgur1, Oktay Unsal1, M Fethullah Aydin1 and Alparslan Ersoy1
1Faculty of Medicine, Department of Nephrology, Uludag University, Bursa, Turkey
2Department of Medical Genetics, Bursa Yuksek Ihtisas Training and Reserach Hospital, Bursa, Turkey
3Department of Nephrology, Bursa Yuksek Ihtisas Training and Reserach Hospital, Bursa, Turkey
- *Corresponding Author:
- Abdulmecit Yildiz
Faculty of Medicine, Department of Nephrology
Uludag University, Bursa, Turkey
E-mail: mecityildiz@gmail.com
Received date: December 10, 2018; Accepted date: December 23, 2018; Published date: December 29, 2018
Citation: Yildiz A, Gorukmez O, Oruc A, Gul CB, Akgur S, et al. (2018) Autosomal Recessive Trait of UMOD Gene in Consanguineous Family Presented with End Stage Renal Disease. J Clin Exp Nephrol Vol.3 No.4: 21. doi: 10.21767/2472-5056.100072
Copyright: © 2018 Yildiz A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
We report a consanguineous family with Uromodulin mutation with autosomal recessive pattern which is not reported before in the literature. Twenty six years of male diagnosed with end stage renal disease of unknown etiology had a history of dialysis in his mother, maternal aunt and his maternal grandfather. In genetic analysis by Sanger sequencing a homozygous missense variation, c.263G>A/ p.Gly88Asp in UMOD (NM_003361) gene has been detected. In summary, we report a novel UMOD mutation in a Turkish family with autosomal recessive trait.
Keywords
End stage renal disease; Sanger sequencing; Genetic disorders
Introduction
Autosomal dominant tubulointerstitial kidney disease (ADTKD) is a group of genetic disorders leading end stage renal disease (ESRD). Mutations in Uromodulin (UMOD) gene encoding uromodulin cause ADTKD also known as uromodulin kidney disease (UKD). Uromodulin is produced in the thick ascending limb (TAL) of the loop of Henle and regulate salt transport. UMOD mutations are generally in autosomal dominant inheritance pattern and almost located on exon 3, 4 or 5. The main alteration is the reduction of the fractional excretion of uric acid due to increased uric acid absorption in the proximal tubule [1,2]. As a result, the clinical features of the disease generally consist of a slowly progressive chronic kidney disease with bland urine and gout history that ended up with ESRD at the 2th-5th decades. Herein we presented a Turkish family with early onset of ESRD at the age of 26 in all affected members as a consequence of an autosomal recessive (OR) mutation in UMOD gene that was not reported before.
Case Report
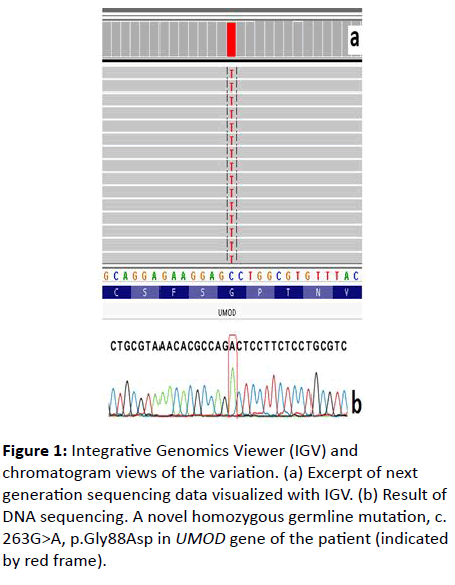
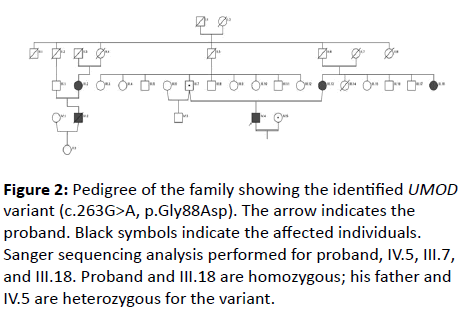
A 26-year-old male was admitted to our hospital because of severe headache. On his initial examination, a blood pressure of 200/110 mmHg was detected. His initial laboratory tests revealed hemoglobin 10.4 g/dL, serum glucose 103 mg/dL, blood urea nitrogen 200 mg/dL, creatinine 8 mg/dL, uric acid 8.8 mg/dL, sodium 142 mEq/L, potassium 4.6 mEq/L, calcium 7.8 mg/dL, phosphorus 6.4 mg/dL, and PTH 775 pg/mL. He had no history of gout or kidney stone diseases. The kidney ultrasonography showed chronic changes in the renal parenchyma, indicating ESRD. Peritoneal dialysis was planned and after catheter insertion, PD treatment was started. Remarkably, the patient had a family history of dialysis commencing at 26 years of age in all affected family members due to ESRD (Figure 1). There was no clinical, laboratory or imaging finding which consistent with monogenic familial kidney diseases like polycystic kidney disease, Alport’s disease or Fabry disease. Therefore, genomic DNA was extracted from peripheral venous blood using the QIAamp® DNA Mini Kit (QIAGEN, Ankara, Turkey). The Custom Panel, Nephropathies Solution (SOPHiA GENETICS), was used to targeted gene enrichments. All procedures were carried out according to the manufacturer’s protocols. It was a capture-based target enrichment kit and covers 44 selected genes related to a broad range of nephropathies. Paired-end sequencing was performed on an Illumina NextSeq 500 system with a read length of 150x2. Base calling and image analysis were conducted using Illumina’s Real- Time Analysis software. The BCL (base calls) binary was converted into FASTQ utilizing Illumina package bcl2fastq. All bioinformatics analysis performed on Sophia DDMTM platform which includes algorithms for alignment, calling SNPs and small indels (Pepper), calling copy number variations (Muskat) and functional annotation (Moka). Raw reads were aligned to the human reference genome (GRCh37/hg19). Variant filtering and interpretation performed on Sophia DDMTM. Integrative Genomics Viewer (IGV) was used to bam file visualization. Next generation sequencing showed a homozygous missense variation, c.263G>A/p.Gly88Asp in UMOD (NM_003361) gene. The variation was confirmed by Sanger sequencing (Figure 2). Sanger sequencing was also applied to relatives of the patient. The variation was homozygous in patients and heterozygous in healthy individuals (Figure 1). This condition showed that the inheritance pattern of the disease was autosomal recessive (OR) [3].
Figure 2: Pedigree of the family showing the identified UMOD variant (c.263G>A, p.Gly88Asp). The arrow indicates the proband. Black symbols indicate the affected individuals. Sanger sequencing analysis performed for proband, IV.5, III.7, and III.18. Proband and III.18 are homozygous; his father and IV.5 are heterozygous for the variant.
Discussion
This variant has not been previously reported in the Human Gene Mutation Database (HGMD; https://www.hgmd.cf.ac.uk/ac/index.php) and in population studies (ExAC: Exome Aggregation Consortium and 1000 Genomes Project). In silico analysis programs (VarSome; DANN Score: 0.9977 and ACMG; Likely Pathogenic) showed that this change could be the cause of the disease. UMOD is located on chromosome 16p12.3-16p13.11 and is composed of 11 exons Knockout mice models proposed that UMOD has extremely important role in regulation of renal outer medullary potassium (ROMK) channel and of sodium–potassium–chloride (NKCC2) transporter in the TAL cells of the kidney. UMOD is also involved in host defense against Escherichia coli [1,2]. Mutation in the UMOD gene causes autosomal dominant tubulointertisial kidney disease, medullary cystic kidney disease type 2 (MCKD2; MIM 603860) and familial juvenile hyperuricemic nephropathy (FJHN; MIM 162000) [4]. Main inheritance of aforementioned UMOD mutations are autosomal dominant pattern, however in our family the inheritance pattern was OR which was not reported before in UMOD related genetic diseases. In UMOD related diseases, hyperuricemia and gout were reported to commonly accompany CKD and renal imaging generally shows cortico medullary cysts. The families carrying this mutation in the literature reach ESRD between the ages of 25 and 70 years or older [5]. Opposite of these findings in our family, none of the affected members had a history of gout and USG revealed only chronic changings without renal cysts. Furthermore, renal replacement treatment initiation age was remarkable younger in all the affected members of our family. Main similarities of our patients with other UMOD related diseases are onset of diseases. Probably concentration defects and decreased secretion of UA were prominent but not reported by patient and his family. Until now to our knowledge autosomal recessive UMOD related disease is not reported.
Conflict of Interest
The authors have declared that no conflict of interest exists.
Informed Consent
A written informed consent was obtained from the patient included in this case study.
References
- Bollee G, Dahan K, Flamant M, Morinière V, Pawtowski A, et al. (2011) Phenotype and outcome in hereditary tubulointerstitial nephritis secondary to UMOD mutations. Clin J Am Soc Nephrol 6: 2429-2438.
- Devuyst O, Olinger E, Rampoldi L (2017) Uromodulin: from physiology to rare and complex kidney disorders. Nat Rev Nephrol 13: 525-544.
- Hart TC, Gorry MC, Hart PS, A Woodard, Z Shihabi, et al. (2002) Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet 39: 882-892.
- Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, et al. (2011) Integrative genomics viewer. Nat Biotechnol 29: 24-26.
- Scolari F, Izzi C, Ghiggeri GM (2015) Uromodulin: from monogenic to multifactorial diseases. Nephrol Dial Transplant 30: 1250-1256.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences