Autosomal Dominant Alport Syndrome: A Case Report of an Increasingly Frequent Alport Syndrome Variant
DOI10.36648/2472-5056.9.4.262
Rosales Montero Alejandro Jose*, Rodriguez Campon Alejandro, Tura Rosales David and Alvarado Gutierez Raul Edilberto
Department of Nephrology, Hospital of Manacor, Balearic Islands, Spain
- *Corresponding Author:
- Rosales Montero Alejandro Jose
Department of Nephrology, Hospital of Manacor, Balearic Islands, Spain
E-mail: alejandrorodriguez25998@gmail.com
Received date: July 30, 2024, Manuscript No. IPJCEN-24-19449; Editor assigned date: August 02, 2024, PreQC No. IPJCEN-24-19449 (PQ); Reviewed date: August 16, 2024, QC No. IPJCEN-24-19449; Revised date: August 23, 2024, Manuscript No. IPJCEN-24-19449 (R); Published date: August 30, 2024, DOI: 10.36648/2472-5056.9.4.262
Citation: Jose RMA, Alejandro RC, David TR, Edilberto AGR (2024) Autosomal Dominant Alport Syndrome: A Case Report of an Increasingly Frequent Alport Syndrome Variant. J Clin Exp Nephrol Vol.9 No.4: 262.
Abstract
Alport syndrome is a hereditary entity that consists of alterations in the structure of collagen type IV that leads to renal and extrarenal compromise. It is clinically characterized by hematuria persistent microscopic and occasionally macroscopic, sensorineural deafness, alterations eye problems (ocular lenticone former) and progression to endstage renal disease. Mutations in the COL4A5 gene are responsible for sex-linked syndrome, which represent 85% of the cases. The remaining 15% corresponds to alport cases of autosomal inheritance (14% recessive and 1% dominant) in which cases intervene the COL4A3 and COL4A4 genes. The clinical characteristics of Alport syndrome linked to sex are the best known, in which the men are affected more frequently and are the ones who progress to End-Stage Renal Disease (ESRD), women are generally carriers. In autosomal forms, the organic commitment is similar but affects men and women the same, the objective of presenting this case was to review and highlight a rare disease to consider in the differential diagnosis of patients with hematuria and kidney failure. The objective of presenting this case was to review and highlight within a disease, which is already rare, where the most frequent type of mutation is the one linked to the X chromosome, a case report of Alport syndrome of autosomal dominant inheritance, in a patient who comes for clinical situation different from clinical manifestations of kidney disease and in which analytical studies and radiological imaging tests (ultrasound and computed axial tomography) reveal advanced chronic kidney disease in which the patient definitively requires renal replacement therapy (hemodialysis). periodically waiting to be included in the kidney transplant waiting list.
Keywords
Alport syndrome; Collagen type IV; Hearing loss; Ocular lenticone former; End-stage renal disease
Introduction
Alport Syndrome (AS) was first described by Cecil Alport in 1927 as a “congenital familial hereditary hemorrhagic nephritis”. Furthermore, it usually affects the eye and cochlea, to different magnitudes, within a wide variety of phenotypes. AS is found in about 3% of children with end-stage renal disease and 0.2% of adults in the United States, with a higher incidence and severity in men than in women. This pathology is the product of an alteration of the basement membranes, secondary to a defect in type IV collagen that usually leads to compromise of the glomerular basement membrane with hematuria. Collagen IV is a class of extracellular matrix protein found ubiquitously in basement membranes of various organs, including kidneys. Six collagen IV a chains, a1(IV) to a6(IV), encoded by COL4A1 to COL4A6 genes, respectively, assemble into three different heterotrimers: Collagen a1a1a2(IV), a3a4a5(IV) and a5a5a6(IV). Collagen a3a4 a5(IV) is the major component of the mature Glomerular Basement Membrane (GBM), though there is a thin layer of collagen a1a1a2(IV) at the GBM’s endothelial aspect. Pathogenic variants in COL4A3, COL4A4, or COL4A5 leading to absence or disruption of the GBM collagen a3a4a5(IV) network cause Alport Syndrome (AS); thus, these will be referred to as alport genes in this article. The “classic” AS presentation is characterized by childhood-onset hematuria, later onset proteinuria, progressive decline in kidney function and Kidney Failure (KF) in adolescence or young adulthood, along with sensorineural hearing loss and eye abnormalities. Because COL4A5 is X-linked, male patients with X-Linked Alport Syndrome (XLAS) typically exhibit more severe symptoms than females with XLAS. In contrast, COL4A3 and COL4A4 are on chromosome 2 and variants cause the more rare Autosomal Recessive Alport Syndrome (ARAS), which affects males and females equally.
Heterozygous COL4A3 and COL4A4 variants have been linked to Thin Basement Membrane Nephropathy (TBMN), also called Benign Familial Hematuria (BFH). However, these are now considered disfavored terms for less severe kidney diseases within the alport spectrum and in some cases have been classified as autosomal forms of AS. Moreover, a variable degree of GBM thinning is in some cases the only early pathologic finding in diseases within the alport spectrum and is quite common, making “thinning of GBM” a nonspecific finding. Therefore, it has been suggested that “thin basement membrane lesion” be used as a term to describe the pathology rather than to justify a diagnosis of TBMN as a specific disease entity. With the increased utilization of molecular genetic testing in clinical practice, pathogenic variants in alport genes have been increasingly reported in patients with diverse clinical presentations, including a more proteinuria-predominant phenotype (nephrotic-range proteinuria or steroid-resistant nephrotic syndrome), kidney failure of unknown etiology, familial Immunoglobulin A (IgA) nephropathy with thin basement membrane and renal cysts in whom polycystic kidney disease has been ruled out. Several studies also consistently reported that pathogenic alport gene variants are the most frequently found genetic abnormalities in adult-onset familial nephrosis with Focal Segmental Glomerulosclerosis (FSGS) lesions. Detection of alport gene variants among patients with diverse clinical presentations challenges the traditional classification of AS/TBMN/BFH/ADAS and newer terms such as “spectrum of Alport syndrome”, “alportrelated nephropathy”, “collagen IV related renal disease” and “collagen IV associated nephropathy” have been used in the literature to denote the kidney disease states linked to pathogenic variants in COL4A3/A4/A5. This review aims to summarize the different clinical manifestations in this disease spectrum and the challenges in categorizing patients due to overlapping and inconsistent presentations and the complex genetics involved. We also put forth the term “Alport kidney disease” to describe nonsyndromic kidney disease resulting from pathogenic variants in the alport genes. Importantly, retention of “Alport” distinguishes COL4A3/A4/A5 nephropathies from the much rarer ones caused by variants in COL4A1 and COL4A2 (Gould syndrome). We hope this term will be considered for adoption by relevant stakeholders, including patients, clinicians, geneticists and scientists.
Below, we present the case report of a patient who comes to our service with a pathological pattern of advanced chronic kidney disease of unknown etiology, with kidney in imaging tests with data suggestive of advanced chronic kidney disease (small size and lost mark of corticomedullary differentiation with the need to start renal replacement therapy (hemodialysis), without significant pathological or surgical history and with negativity in serological studies for infectious and autoimmune diseases, in which, thanks to the massive genetic sequence study (known in our department as Renal Exome Panel) a homozygous mutation is detected in the COL4A3 gene, in the form of autosomal dominant inheritance, establishing a "homozygous autosomal dominant inheritance Alport syndrome" as the cause of chronic kidney disease.
Case Presentation
Clinical history and follow-up
22-year-old patient, women, native of Morocco, without medical-surgical history and without known usual underlying treatment. No history of consumption of NSAIDs or any other nephrotoxic drug. No history of smoking, alcoholism or psychoactive drug use.
Family medical history
Father and paternal grandmother of nephropathy whose cause cannot be specified. Younger sister who is being studied in the Pediatrics department for alterations in urinary sediment (microhematuria).
We present the case report of a female patient who went to the emergency department due to trauma to the right rib area due to a traffic accident (fall as a result of a collision with a car) and in which insufficiency was detected in the analysis. Severe kidney disease (serum creatinine of 5.79 mg/dl, previous analysis of 3,4 and 5 years ago with creatinine levels within the normal range), microscopic hematuria (10-20 red cells per high-power field) not present in previous urine tests and proteinuria of 3 g in 24 h urine with albuminuria of 1970 mg in 24 h not present in urine tests either previous. In which in the computed axial tomography of the abdominopelvic area and in the renal and urinary tract ultrasound, small kidneys (7 cm) were observed, with complete loss of corticomedullary differentiation, so biopsy was ruled out. Renal, profile of autoimmunity studies, serum complement, serum proteinogram and normal immunoglobulins. Only lupus anticoagulant stands out, but with negative antiphospholipid antibodies (cardiolipin and beta-2glycoprotein-I) and serology without alterations are observed. Due to nonrecovery of renal function and appearance of uremic symptoms, renal replacement therapy (hemodialysis) was initiated through a temporary right femoral catheter and subsequently, given the sustained situation of end-stage renal disease, a right jugular tunneled permanent catheter was placed to continue the hemodialysis program. periodically, 3 sessions per week. A diagnosis of nephropathy of unknown etiology was established and a massive genetic sequence study was requested, determining the presence of a mutation in homozygous pathogenic variation of an amino acid change (see below) in the COL4A3 gene with an autosomal dominant pattern in homozygosity, establishing a diagnosis of kidney disease. terminal chronic secondary to Alport syndrome of autosomal dominant homozygous inheritance. Currently pending inclusion on the waiting list for kidney transplant. In a color Doppler echocardiogram study, no alterations were detected. An ophthalmology study was also requested to rule out alterations in the crystalline area, with the same negative results and an audiometric study that was also normal.
Given the non-recovery of kidney function, it was decided to place a right jugular tunneled catheter and establish a periodic hemodialysis regimen of 3 weekly sessions.
Discussion
Alport syndrome (previously known as hereditary nephritis) and is considered and is considered the second most common inherited kidney disease after autosomal dominant polycystic kidney disease. In recent years, different opinions have been published on the nomenclature of familial hematuria, making the term “benign familial hematuria” obsolete, since it has been seen that some cases progress to Renal Replacement Therapy (RRT), thus eliminating the character of "benign". For a long time, the term “thin basement membrane disease” has also been used, being a histological concept that does not consider genetics or clinical features and there are also other entities that can present it. The clinical presentation is very variable: From asymptomatic patients or with microhematuria, to bilateral sensorineural hearing loss, ocular involvement, proteinuria and progression to Sleep Disordered Breathing (SDB). In the case at hand, our patient presents a pathogenic variant of the COL4A3 gene of autosomal dominant inheritance in a homozygous form and evolution of chronic kidney disease to an advanced stage before the age of 30, which suggests that there could be environmental factors that would favor the high penetrance of the disease.
Genetics
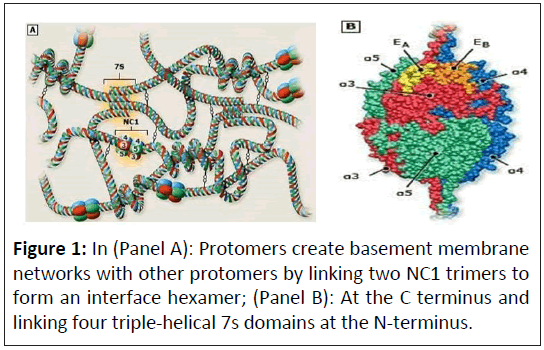
Alport syndrome is a primary basement membrane disorder that arises from pathogenic variants in genes encoding several members of the collagen IV protein family. Collagen IV molecules are composed of three alpha chains that form triple helical structures through specific interactions of C-terminal noncollagenous domains [1]. Six distinct chains of type IV collagen are encoded by six different genes that are distributed in headto- head pairs on three chromosomes (Figure 1).
The genes include:
• COL4A1 and COL4A2 in 13q34
• COL4A3 and COL4A4 in 2q35-37
• COL4A5 and COL4A6 on the X chromosome
The six alpha chains of collagen IV form three triple helical protomers: Alpha-1-1-2, alpha-3-4-5 and alpha-5-5-6. These protomers are organized into networks by end-to-end connections through C-terminal and N-terminal interactions. Genetic analyzes of affected families have identified the affected genes for the four different modes of transmission observed in patients with Alport syndrome, which are discussed in the following sections [2]:
• X-linked
• Autosomal recessive
• Autosomal dominant
• Digenic
Pathogenic variants in the COL4A3, COL4A4 and COL4A5 genes disrupt the synthesis and/or formation of collagen IV alpha-3-4-5 protomers and networks. Based on pedigree studies, linkage analysis and Sanger sequencing, the relative frequencies of the four genetic types were estimated at 80% for X-linked disease, 15% for autosomal recessive disease, less than 5% for autosomal dominant disease and case reports for digenic inheritance. However, studies using next generation sequencing in Alport families suggest that autosomal dominant Alport syndrome may occur more frequently (20 to 30 percent of patients) than previously thought.
X-Linked Alport Syndrome (XLAS)
A few years ago it was considered the most common form because it had a classic presentation with kidney involvement at an early age, sensorineural hearing loss and ocular alterations. It is caused by pathogenic variants in the COL4A5 gene. Male patients with XLAS usually present with hearing loss and progress to Sensorineural Deafness Bilateral (SDB) in the second or third decade of life. While around 12% of women with XLAS can progress to SDB before the age of 40, increasing to 30 and 40% at 60 and 80 years respectively [3]. Affected men do not transmit the disease to their sons, however they have a 100% probability of transmitting it to their daughters. Women with XLAS have a 50% chance of having affected offspring. The variable course in females is probably due to lionization, by which only one X chromosome is active per cell. As a result, in most women with X-linked Alport syndrome, approximately half of the cells will express the variant COL4A5 allele and the remaining cells the normal COL4A5 allele, leading to a variable phenotype that is usually less severe than in affected men.
Autosomal Recessive Alport Syndrome (ARAS)
Patients with ARAS have 2 pathogenic variants inherited from both parents in the COL4A3 or COL4A4 genes. The COL4A3 and COL4A4 genes encode the alpha-3(IV) chain (containing the Goodpasture antigen) and the alpha-4(IV) chain, respectively.
Patients with ARAS, both men and women, usually develop hearing loss and progress to SDB before the age of 30. The parents have an autosomal dominant inheritance pattern and may be clinically asymptomatic, present microhematuria or present SDB at usually advanced ages [4]. Currently, for experts it is not clear whether these patients with a COL4A3 or COL4A4 variant, being asymptomatic, could be considered as autosomal dominant alport or would only be a risk factor for chronic kidney disease, given the high frequency of these variants in the population general [5].
Autosomal Dominant Alport Syndrome (ADAS)
Patients with ADAS have a pathogenic variant in the COL4A3 or COL4A4 gene and the clinical presentation is as varied as microhematuria only or evolving to SDB at advanced ages, generally after 40 years. Ocular and auditory involvement is much less common than in XLAS and ARAS [6,7].
In 2021, a cohort of 252 patients with a diagnosis of ADAS has been published, where it has been observed that the age of renal survival was 67 years, extrarenal affections were very rare and highlighting that of the patients who did not have proteinuria, none of them progressed to chronic kidney disease [8].
Recently, a study was published on the prevalence of variants in COL4A3 and COL4A4 in the general population without known kidney disease, highlighting that 1/106 individuals may have one of these variants. Importantly, the pathogenicity of these genetic variants must be adjusted to the penetrance of the disease and the individual variants. The penetrance of the disease may also depend on other genetic and environmental factors. Therefore, calling all individuals who have one of these variants in COL4A3 or COL4A4 autosomal dominant Alport syndrome is not very clear, since it could only be a risk factor without yet being able to explain why such a low percentage of those individuals evolve to SDB.
Alport with digenic inheritance
In recent years, cases of AS with digenic inheritance have been described, that is, with pathogenic variants in 2 type IV collagen genes at the same time, which could justify a more severe clinical presentation, but this premise is not met in all series published [9].
Alport syndrome with digenic inheritance is due to a pathogenic variant in COL4A5 plus one in COL4A3 or COL4A4 or by a pathogenic variant in COL4A3 plus one in COL4A4. These will be considered separately due to their population frequencies, modes of inheritance and because their clinical and molecular consequences are different. For digenic variants affecting COL4A5 plus COL4A3 or COL4A4, many of the characteristics depend on whether the COL4A5 variant affects a male or female and in all patients, the severity variant also contributes to the phenotype [10].
Contiguous genes syndrome associated with Xlinked Alport syndrome
“Contiguous genes” syndrome is caused by a deletion of the 5' end of the COL4A5 gene that includes the COL4A6 gene. It is characterized by presenting genital, uterine and esophageal leiomyomatosis associated with XLAS [11].
Pathogenesis
The elucidation of the pathogenesis of alport syndrome was facilitated by the chance observation that the Glomerular Basement Membrane (GBM) of most affected patients did not bind to antibodies from patients with anti-GBM antibody disease (including Goodpasture syndrome) [12]. This finding suggested an abnormality in collagen IV, the target protein of anti-GBM antibodies. Alpha-3, alpha4 and alpha-5 (IV) chains are highly expressed and codistributed within normal GBM. They form a collagen IV network within the GBM that is distinct from that formed by the alpha-1 (IV) and alpha-2 (IV) chains. Pathogenic genetic variants affecting alpha-3, alpha-4 and alpha-5 (IV) chains impair their deposition in this collagen network, leading to secondary changes in the GBM and resident glomerular cells that predispose to development of glomerulosclerosis. Abnormal expression of collagen IV alpha-3-4-5 networks in the basement membranes of the eye and cochlea results in specific ocular abnormalities and sensorineural hearing loss.
Monoclonal antibody probes have been used to determine the tissue distribution of alpha-3, alpha-4 and alpha-5 (IV) chains in normal tissues and tissues from patients with Alport.
Collagen IV chains are normally located in Bowman's capsule and in the basement membranes of the glomerulus, the distal and collecting tubules and the basement membranes of the cochlea and eye. Therefore, an abnormality in any of these chains can impair the integrity of the basement membranes at these sites, leading to the various clinical findings of alport syndrome. In most patients with alpha-5(IV) variants, alpha-3, alpha-4 and alpha-5(IV) chains are absent from the GBM [12]. However, transcription of the alpha-3(IV) and alpha-4(IV) genes is not inactivated in the renal cortex, suggesting that failure to incorporate these chains is responsible for the lack of glomerular expression and not from synthesis failure [13].
In normal individuals, the alpha-5(IV) chain is present in the underlying basement membrane of the epidermis as a component of the alpha-5-5-6 networks. In individuals with variants in the COL4A5 gene on the X chromosome (i.e., X-linked inheritance), there is a complete absence of alpha-5(IV) chain within the epidermal basement membranes in the majority of affected males.
Diagnosis
The diagnostic algorithm is illustrated in (Figure 1).
Genetic study
The definitive diagnosis is genetic study, reducing the risk of complications from diagnosis by kidney biopsy. The index case is usually studied using a gene panel or exome and then family segregation is carried out, that is, the pathogenic variant found in the rest of the available relatives is checked [14].
Once the pathogenic variant is identified, genetic counseling should be performed. Those patients with gestational desire should be offered the possibility of prenatal and preimplantation diagnosis.
Genetic study is indicated in all cases of suspected AS.
Pathology
Pathology of Alport syndrome is speci ied by the following:
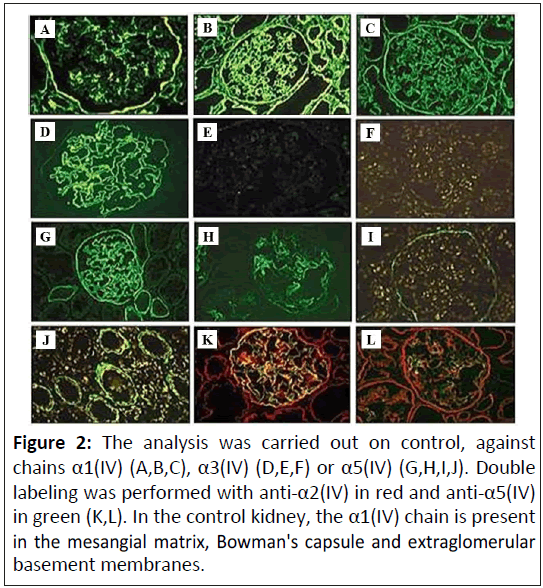
Kidney immunostaining: Immunostaining of renal biopsy specimens for collagen IV may be diagnostic for patients with suspected Alport syndrome (Figure 2). (A,D): In control kidney, the α1(IV) chain is present in the mesangial matrix, Bowman's capsule and the extraglomerular basement membranes; (B and C): The α3(IV) and α5(IV) chains are distributed within the GBM. The Bowman's capsule is strongly α5(IV)-positive en C; (E,I): In Xlinked Alport syndrome, no α3(IV) expression was detected in a male patient (id for α4-α5). In both types of Alport syndrome (XLAS and ARAS), α1(IV) is diffusely expressed in the GBM; (G,H): In X-linked Alport syndrome, the distribution is segmental in a female patient; (I,J,K,L): In autosomal recessive Alport syndrome, no α3(IV)-α5(IV) labeling is detected in the GBM; (J): Whereas α5(IV) is expressed in Bowman's capsule; (K): And the basement membranes of the collecting ducts (L).
X-linked disease: Men with X-linked Alport syndrome usually show a complete absence of immunostaining for alpha-3, alpha-4 and alpha-5(IV) chains in the kidneys, whereas they present an irregular loss of staining in the GBM and tubular basement membranes.
As with alpha-5(IV) chain skin staining, approximately 20% of men with X-linked Alport syndrome have normal staining of the kidney basement membranes for alpha-3, alpha -4 and alpha5 (IV). Further quantitative analysis reveals lower amounts of alpha-3, alpha-4 and/or alpha-5(IV) chains in these cases compared to healthy controls. Some of these patients have missense variants of COL4A5, which may explain the detection of alpha IV chains with immunostaining, although with lower intensity [15].
Autosomal recessive disease: People with autosomal recessive Alport syndrome have abnormalities in collagen IV expression that differ from those in patients with X-linked disease. These patients usually have a complete absence of staining for alpha chains. Alpha-3 and alpha-4(IV).
However, while their Glomerular Basement Membranes (GBMs) do not show staining for the alpha-5(IV) chain, Bowman's capsules and distal tubular basement membranes do stain for the alpha5(IV) chain. This observation can be interpreted as a failure of the alpha-5(IV) chain to be deposited in the GBM due to the absence of the alpha-3 and alpha-4(IV) chains, but the alpha-5(IV) chain, together with the alpha-6(IV) chain, it is deposited in the basement membranes of the distal tubule, Bowman's capsules and the epidermis [16].
Kidney and skin biopsy
Currently, kidney or skin biopsy is not routinely performed for diagnosis. Histologically, the renal biopsy is characterized by presenting thinning of the thin basement membrane under the electron microscope. Under the light microscope, the findings can be normal, present as mesangial proliferation with nonspecific deposits of IgM and C3, or in more advanced stages such as focal and segmental glomerulosclerosis [17]. For those cases in which the clinical evolution is not typical of AS, with unexpected deterioration in renal function, a renal biopsy would be justified. Skin biopsy is not currently considered useful.
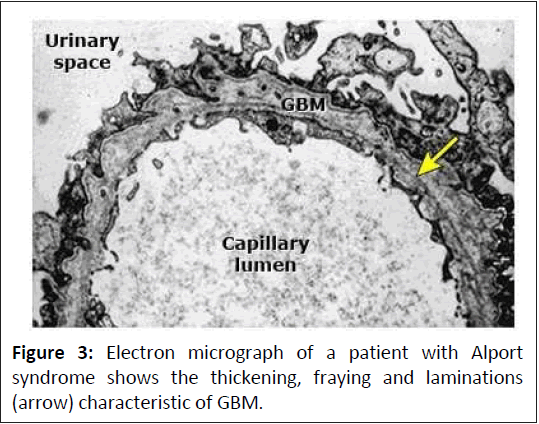
Histological changes: Changes on light microscopy are nonspecific and include focal increases in glomerular cellularity, progressing to glomerulosclerosis and interstitial fibrosistubular atrophy over time and an interstitial infiltrate containing lipid-laden foam cells. Typically, histological changes in Alport syndrome increase in severity with age.
The earliest ultrastructural lesion is thinning of the GBM. Over time, a longitudinal unfolding of the lamina dense of the GBM develops, producing a laminated appearance that is diagnostic of Alport syndrome. In men with X-linked Alport syndrome, the proportion of GBM showing division increases from approximately 30% at 10 years to more than 90% at 30 years. A similar progression is likely to occur in patients with autosomal recessive Alport syndrome. Women with X-linked Alport syndrome and men and women with autosomal dominant Alport syndrome, may have thin GBM or a mixture of thin and lamellar GBM.
Renal biopsy of affected individuals at an early age may reveal only nonspecific light microscopic changes and not definitive electron microscopy findings. However, immunostaining results for collagen IV alpha chains are frequently diagnostic even in the absence of specific ultrastructural changes. In some patients, less invasive skin biopsy with appropriate immunohistochemical analysis may be the preferred diagnostic study [18].
Skin: Immunohistochemical studies with a monoclonal antibody directed against the alpha-5(IV) chain demonstrate the complete absence of the alpha-5(IV) chain within the epidermal basement membranes in the majority of men with Alport syndrome linked to chromosome X, while female carriers have discontinuous staining (Figure 2). This latter observation is compatible with lyonization in female carriers, in whom half of their cells would be expected to express a normal alpha-5(IV) chain. However, conventional immunofluorescence microscopy will detect the alpha5(IV) chain from the skin in about 20% of men with X-linked Alport syndrome and 30%-40% of heterozygous women. All patients with autosomal recessive and autosomal dominant Alport syndrome have normal skin reactivity for alpha-5(IV) (Figure 3). Therefore, the presence of epidermal basement membrane staining for alpha-5(IV) does not exclude the diagnosis of X-linked or autosomal Alport syndrome. However, the absence of alpha-5(IV) chain in a skin biopsy is diagnostic of X-linked Alport syndrome [19].
Figure 2: The analysis was carried out on control, against chains α1(IV) (A,B,C), α3(IV) (D,E,F) or α5(IV) (G,H,I,J). Double labeling was performed with anti-α2(IV) in red and anti-α5(IV) in green (K,L). In the control kidney, the α1(IV) chain is present in the mesangial matrix, Bowman's capsule and extraglomerular basement membranes.
Clinical manifestations
Clinical manifestations are mentioned as below:
Kidney involvement: Hematuria is the most important sign, whether persistent or intermittent microhematuria or episodes of macroscopic hematuria associated with respiratory infections. In addition, they may present proteinuria that increases with age and may progress to nephrotic syndrome and SDB. The degree of kidney involvement presents great intrafamilial variability. The renal replacement treatment of choice is kidney transplantation. Some cases of transplant patients with AS who developed anti- GBM Ab with loss of the kidney graft have been described, but with the advance of immunosuppressive treatments, this percentage has decreased from 1%-5% to 0.4% in recent records [20].
Eye involvement: Ocular involvement appears in 15%-40% of cases. Bilateral anterior lenticonus is the practically pathognomonic lesion. It appears during the second decade of life, does not cause vision loss and does not require follow-up. In addition, they may present lesions in the cornea, lens and retina (cataracts, corneal erosions, retinal flaws). Generally, an annual control is carried out depending on the type of pathology [21]. Hearing impairment: Sensorineural hearing loss is not congenital; it usually appears in late childhood or early adolescence. The diagnosis is made with audiometry and the involvement is bilateral for highpitched sounds (frequency 4000-8000 Hz). It tends to be more common in adolescence in patients with XLAS and ARAS.
MYH9 nephropathy: Pathogenic variants in the MYH9 gene produce a clinical picture that is characterized by the presence of macrothrombocytopenia, leukocyte inclusions and a variable risk of developing microhematuria, renal failure, hearing loss and cataracts in youth or adulthood. It is transmitted with an autosomal dominant inheritance pattern. The group of diseases caused by pathogenic variants in the MYH9 gene were grouped into four syndromes characterized by presenting macrothrombocytopenia associated with other diseases. Historically, two of these syndromes, Fechtner syndrome and Epstein syndrome, presented renal involvement and were considered variants of Alport syndrome [22].
HANAC syndrome: It is caused by pathogenic variants in the COL4A1 gene with an autosomal dominant inheritance pattern. Clinically it is characterized by the association of hematuria, with or without proteinuria, retinal hemorrhages due to tortuosities in the retinal arteries, cardiac arrhythmia, Raynaud's phenomenon and muscle contractures. Unlike other forms of familial hematuria, the published cases presented non-glomerular anomalies and at a histological level the thickness of the basement membrane is usually normal, as well as the expression of type IV collagen chains [23].
Differential diagnosis: Alport syndrome is often differentiated from other major causes of persistent glomerular hematuria by a positive family history of hematuria associated with renal failure and deafness. Other glomerular disorders that occur in children with microscopic hematuria include IgA nephropathy, in which the family history is usually negative and "Thin Basement Membrane Nephropathy" (TBMN), in which the family history may be positive for hematuria, but renal failure and deafness are usually absent or occur relatively late in life. However, some experts in the field, including the author, consider TBMN to be an autosomal dominant Alport syndrome, as these patients typically have heterozygous variants in the COL4A3 or COL4A4 genes.
As noted above, the diagnosis of Alport syndrome is differentiated from other glomerular diseases by confirmatory skin or kidney biopsy or molecular genetic testing. Alport syndrome is distinguished by the presence of the characteristic finding of Glomerular Basement Membrane (GBM) lamination in renal biopsy specimens or collagen IV abnormalities by immunostaining or by the identification of pathogenic variants in COL4A3, COL4A4, or COL4A5. As noted above, in this author's opinion, thin GBMs with or without focal segmental glomerulosclerosis in a patient with a COL4A3, COL4A4, or COL4A5 variant are correctly diagnosed as Alport syndrome.
Megathrombocytopenia (thrombocytopenia with large or giant platelets) has been described in some families with autosomal dominant glomerulopathy and sensorineural deafness. This complex has been called Epstein syndrome or Fechtner syndrome when associated with cytoplasmic inclusions of leukocytes. These disorders have been mapped to chromosome 22 and are the result of variants in the gene encoding non-muscle myosin heavy chain 9 (MYH9). Variants of this gene can also cause Sebastian syndrome, another giant platelet disorder and hereditary nonsyndromic deafness [24].
Prognosis: In the X-linked disease form, the most common type of Alport syndrome, about 50% of males require dialysis or kidney transplantation by 30 years and approximately 90% develop ESRD before 40. Female patients with X-linked Alport syndrome have a better prognosis, with about 12% developing End-Stage Renal Disease (ESRD) by age 40. However, studies indicate significant renal morbidity in females with proteinuria and hearing impairment.
By age 60, this rate increases to about 30% and by 60 years of age, the rate of ESRD approaches 40%. In the female population, proteinuria and hearing loss are risk factors for the progression to ESRD. In comparison, the autosomal recessive form of Alport syndrome can cause kidney failure by age 20. In contrast, the autosomal dominant form of the disease typically has a delay in ESRD until middle age [25].
Deterrence and patient education: Patients should be given information leaflets regarding symptoms of renal failure and its systemic effects. Parents of affected children should be knowledgeable about the signs and symptoms of the disease that warrant hospital admission. Family members of patients with Alport syndrome should consult their primary care providers to go through screening as per the local guidelines. Patients with Alport syndrome should undergo genetic counseling before getting married and having children. There are various support groups available that can help patients and their families have better handling of their circumstances.
Treatment and follow-up: Hematuria does not require treatment. But when albuminuria/proteinuria appears, it is necessary to start treatment with inhibitors or antagonists of the renin angiotensin aldosterone system to delay its evolution to TRS. Several studies have shown that RAS blockade therapy reduces proteinuria and decreases the rate of glomerulosclerosis and disease progression in patients with Alport syndrome. Additional data suggest that early RAS blockade therapy is beneficial and safe in patients who have microalbuminuria but have not yet developed overt proteinuria. As a result, the Alport syndrome research collaborative’s clinical practice recommendations were updated to initiate earlier intervention. These guidelines recommend initiation of RAS blockade at the time of diagnosis in men with X-linked Alport syndrome and men and women with autosomal recessive Alport syndrome, regardless of whether microalbuminuria or proteinuria is present and in women with Xlinked Alport syndrome and men and women with autosomal dominant Alport syndrome if persistent microalbuminuria is present.
In a large study of patients with Alport syndrome followed by the European alport registry for a mean duration of more than 20 years, initiation of ACE inhibitors was found to delay dialysis in patients with proteinuria and normal renal function in compared to those who never received such therapy or who received treatment only when they developed impaired renal function (dialysis initiated at a mean age of 40, 22 and 25 years, respectively). In a subsequent prospective report, RAS blockade therapy was also effective in preventing progression in renal function decline in patients with autosomal or X-linked Alport syndrome with heterozygous mutations in type IV collagen genes.
In a large retrospective study of Japanese patients with Xlinked disease, treatment with RAS blockade therapy was associated with a delay in the onset of renal failure (previously referred to as end-stage renal disease). Exposure to RAS blocking therapy vs no exposure delayed renal failure with truncating (median age 28 vs. 16 years) and non-truncating (median age 50 vs. 33 years) mutations.
Currently there is no specific treatment for AS, but several studies are underway with different drugs such as anti- miRNA21, STAT3 inhibitors, estimated Glomerular Filtration Rate (eGFR) inhibitors, paricalcitol that would act by reducing the degree of renal fibrosis and its evolution to SDB. At the moment, the FDA has not approved bardoxolone for patients with Alport syndrome because it has not shown sufficient benefit to slow the deterioration of kidney function. The Dapagliflozin and Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) clinical trial suggests a beneficial effect of Sodium-Glucose Transport Protein 2 (SGLT2) inhibitors in Chronic Kidney Disease (CKD) of nonmetabolic origin, where the number of patients included with Alport syndrome was low and, therefore, the conclusions cannot be extrapolated to this population at this time and its study should be expanded. The EMPA-KIDNEY study was recently published in which it was observed that empaglifozin slows the decline in glomerular filtration in all subgroups with albuminuria, so SGLT2 inhibitors could be beneficial for Alport syndrome. The observational study “Guard Alport” will be carried out by Prof. Oliver Gross, where the effect of Sodium-Glucose Linked Transporter (SGLT) inhibitors will be studied in patients diagnosed with Alport syndrome (NCT02378805). Therapies with stem cells that would act at the level of the glomerular basement membrane and chaperones that could act directly on the triple helix of the collagen IV chain are in the research stage.
In the case of our patient, there was no possibility of treatment improvement with the strategy of Renin-Angiotensin-Aldosterone System (RAAS) blockade and SGLT inhibitors because she already presented with advanced kidney disease [26-29].
Conclusion
The genetic and clinical complexities of Alport syndrome and the related alport kidney diseases make the classification of patients with less severe manifestations difficult and subjective. These patients are much more numerous than those with classic severe AS, so there is an urgency to ensure that they receive appropriate attention and care from nephrologists even if the risk of KF is low. Although the working group recommended classifying patients based on mode of inheritance, in this review we proposed a more detailed patient classification across the disease spectrum by severity of AS phenotypes, from the classic severe AS (XLAS in males and ARAS), in which there is no intact collagen α3α4α5(IV) in the GBM, to hematuric Alport kidney disease, the most favorable prognosis with only a slightly increased risk of KF over the general population. We also emphasized those patients with a proteinuria-predominant Alport kidney disease presentation (Group 3) who might be diagnosed as FSGS and given unnecessary immunosuppressive therapy. It is now clear that a variant in COL4A3, COL4A4 and COL4A5 is a risk factor for CKD. Whether one thinks of people with these variants as having a single disease with a spectrum of phenotypes (Alport syndrome) or as having distinct disorders (Alport syndrome or thin basement membrane nephropathy), it is important that they have regular follow-up by a primary provider or nephrologist and initiation of ACE-inhibitor therapy when appropriate. It is likewise vital that clinicians attempt to establish a definitive diagnosis in people with persistent glomerular hematuria. Individuals diagnosed with Alport syndrome or found to have COL4A variants should be informed by clinicians about Alport syndrome registries to help further our understanding of the disease and responses to intervention.
References
- Miner JH, Baigent C, Flinter F, Gross O, Judge P, et al. (2014) The 2014 international workshop on Alport syndrome. Kidney Int 86: 679-684.
[Crossref] [Google Scholar] [Indexed]
- Savige J, Rana K, Tonna S, Buzza M, Dagher H, et al. (2003) Thin basement membrane nephropathy. Kidney Int 64: 1169-1178.
[Crossref] [Google Scholar] [Indexed]
- Kashtan CE, Ding J, Garosi G, Heidet L, Massella L, et al. (2018) Alport syndrome: A unified classification of geneticdisorders of collagen IV α345: A position paper of the Alport syndrome classification working group. Kidney Int 93: 1045-1051.
[Crossref] [Google Scholar] [Indexed]
- Matthaiou A, Poulli T, Deltas C (2020) Prevalence of clinical, pathological and molecular features of glomerular basement membrane nephropathy caused by COL4A3 or COL4A4 mutations: A systematic review. Clin Kidney J 13: 1025-1036.
[Crossref] [Google Scholar] [Indexed]
- Gibson J, Fieldhouse R, Chan MMY, Sadeghi-Alavijeh O, Burnett L, et al. (2021) Prevalence estimates of predicted pathogenic COL4A3-COL4A5 variants in a population sequencing database and their implications for Alport syndrome. J Am Soc Nephrol 32: 2273-2290.
[Crossref] [Google Scholar] [Indexed]
- Li Y, Groopman EE, D’Agati V, Prakash S, Zhang J, et al. (2020) Type IV collagen mutations in familial IgA nephropathy. Kidney Int Rep 5: 1075-1078.
[Crossref] [Google Scholar] [Indexed]
- Sevillano AM, Gutierrez E, Morales E, Hernandez E, Molina M, et al. (2014) Multiple kidney cysts in thin basement membranedisease with proteinuria and kidney function impairment. Clin Kidney J 7: 251-256.
[Crossref] [Google Scholar] [Indexed]
- Gulati A, Sevillano AM, Praga M, Gutierrez E, Alba I, et al. (2020) Collagen IV gene mutations in adults with bilateral renalcysts and CKD. Kidney Int Rep 5: 103-108.
[Crossref] [Google Scholar] [Indexed]
- Mencarelli MA, Heidet L, Storey H, van Geel M, Knebelmann B, et al. (2015) Evidence of digenic inheritance in Alport syndrome. J Med Genet 52: 163-174.
[Crossref] [Google Scholar] [Indexed]
- Savige J, Harraka P (2021) Pathogenic variants in the genes affected in Alport syndrome (COL4A3-COL4A5) and their association with other kidney conditions: A review. Am J Kidney Dis 78: 857-864.
[Crossref] [Google Scholar] [Indexed]
- Kruegel J, Rubel D, Gross O (2021) Alport syndrome-insights from basic and clinical research. Nat Rev Nephro l9: 170-178.
[Crossref] [Google Scholar] [Indexed]
- Thomas CP, Mansilla MA, Sompallae R, Mason SO, Nishimura CJ, et al. (2017) Screening of living kidney donors for genetic diseases using a comprehensive genetic testing strategy. Am J Transplant 17: 401-410.
[Crossref] [Google Scholar] [Indexed]
- Rheault MN, Kashtan CE (2017) Alport syndrome and thin basement membrane nephropathy. Pediatric Kidney Disease, Second Edition, pp: 499-514.
- Furlano M, Martínez V, Pybus M, Arce Y, Crespí J, et al. (2021) Clinical and genetic features of autosomal dominant Alport syndrome: A cohort study. Am J Kidney Dis 78: 560-570.e1.
[Crossref] [Google Scholar] [Indexed]
- Fallerini C, Baldassarri M, Trevisson E, Morbidoni V, Manna AL, et al. (2017) Alport syndrome: Impact of digenic inheritance in patients management. Clin Genet 92: 34-44.
[Crossref] [Google Scholar] [Indexed]
- Deltas C (2018) Digenic inheritance and genetic modifiers. Clin Genet 93: 429-438.
[Crossref] [Google Scholar] [Indexed]
- Daga S, Fallerini C, Furini S, Pecoraro C, Scolari F, et al. (2019) Non-collagen genes role in digenic Alport syndrome. BMC Nephrol 20: 70.
[Crossref] [Google Scholar] [Indexed]
- Savige J, Renieri A, Ars E, Daga S, Pinto AM, et al. (2022) Digenic Alport syndrome. Clin J Am Soc Nephrol 17: 1697-1706.
[Crossref] [Google Scholar] [Indexed]
- Gubler MC (2008) Inherited diseases of the glomerular basement membrane. Nat Clin Pract Nephro l4: 24-37.
[Crossref] [Google Scholar] [Indexed]
- Shah SN, Weinberg DV (2002) Giant macular hole in Alport syndrome. Ophthalmic Genet 17: 70-74.
[Crossref] [Google Scholar] [Indexed]
- Uliana V, Marcocci E, Mucciolo M, Meloni I, Izzi C, et al. (2011) Alport syndrome and leiomyomatosis: The first deletion extending beyond COL4A6 intron 2. Pediatr Nephrol 26: 717-724.
[Crossref] [Google Scholar] [Indexed]
- Stam AH, Kothari PH, Shaikh A, Gschwendter A, Jen JC, et al. (2016) Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Brain 139: 2909-2922.
[Crossref] [Google Scholar] [Indexed]
- Kashtan CE (2021) Alport syndrome: Achieving early diagnosis and treatment. Am J Kidney Dis 77: 272-279.
[Crossref] [Google Scholar] [Indexed]
- Chavez E, Goncalves S, Rheault MN, Fornoni A (2024) Alport syndrome. Adv Kidney Dis Health 31: 170-179.
- Omachi K, Miner JH (2019) Alport syndrome therapeutics: Ready for prime-time players. Trends Pharmacol Sci 40: 803-806.
[Crossref] [Google Scholar] [Indexed]
- Torra R, Furlano M (2019) New therapeutic options for Alport syndrome. Nephrol Dial Transplant 34: 1272-1279.
[Crossref] [Google Scholar] [Indexed]
- Kashtan CE (2022) What the adult nephrologist should know about Alport syndrome. Adv Chronic Kidney Dis 29: 225-230.
[Crossref] [Google Scholar] [Indexed]
- Mabillard H, Sayer JA (2020) SGLT2 inhibitors-a potential treatment for Alport syndrome. Clin Sci (Lond) 134: 379-388.
[Crossref] [Google Scholar] [Indexed]
- Savva I, Pierides A, Deltas C (2016) RAAS inhibition and the course of Alport syndrome. Pharmacol Res 107: 205-210.
[Crossref] [Google Scholar] [Indexed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences