Application of Point-of-Care Ultrasound (POCUS) in Children with End-Stage Kidney Disease
Ahmet Yontem*, Dincer Yildizdas
DOI10.36648/2472-5056.21.s2.004
Ahmet Yontem* and Dincer Yildizdas
Division of Pediatric Intensive Care Unit, Cukurova University, Adana, Turkey
- *Corresponding Author:
- Ahmet Yontem
Division of Pediatric Intensive Care Unit,
Cukurova University,
Adana,
Turkey,
E-mail: drayontem@gmail.com
Received Date: July 09, 2021; Accepted Date: July 23, 2021; Published Date: July 30, 2021
Citation: Yontem A, Yildizdas D (2021) Application of Point-of-Care Ultrasound (POCUS) in Children with End-Stage Kidney Disease. J Clin Exp Nephrol Vol. 6 No.S2: 004.
Abstract
The use of point-of-care ultrasound is becoming increasingly common among non-radiologist clinicians. There has also been increased interest in the bedside assessment of fluid status in hemodialytic patients. Lung ultrasound and inferior vena cava collapsibility index measurement methods may assist the detection of fluid overload. Current information on the role of POCUS in regard of the assessment of predialytic fluid overload in hemodialytic children is scarce. In this article, we aim to review and emphasize the importance of a non-invasive bedside method of assessing the predialytic volume overload of children based on the published literature. Well-designed studies with larger samples are needed to prove the reliability of the use of lung ultrasound and inferior vena cava collapsibility index in the assessment of predialysis fluid overload in children.
Keywords
Fluid predialytic; ESKD; Inferior vena cava; Lung ultrasound; POCUS
Introduction
Point-of-care ultrasound (POCUS) is compact, relatively inexpensive and portable equipment that enables image acquisition and interpretation to done by the clinician at the bedside. Thus, scanning results can be immediately acted upon and integrated into comprehensive treatment management.
The use of portable bedside ultrasonography is becoming increasingly common among non-radiologist clinicians. It has also been used in operation rooms and postoperative care. The Society of Hospital Medicine, the American College of Physicians, the Alliance for Academic Internal Medicine, and the European Society of Paediatric and Neonatal Intensive Care, have also endorsed its use [1-4]. Today, emergency and intensive care clinicians most commonly use POCUS in the management of the treatment, and the main areas of use are thoracic, cardiac, abdominopelvic, and vascular examinations. Fluid status, in particular, is an area of interest for POCUS, as it can guide the management of the patient. There has been increased interest in the bedside assessment of fluid status in hemodialytic patients. However, further studies with data are potentially needed to integrate POCUS in the management of children at hemodialysis centres.
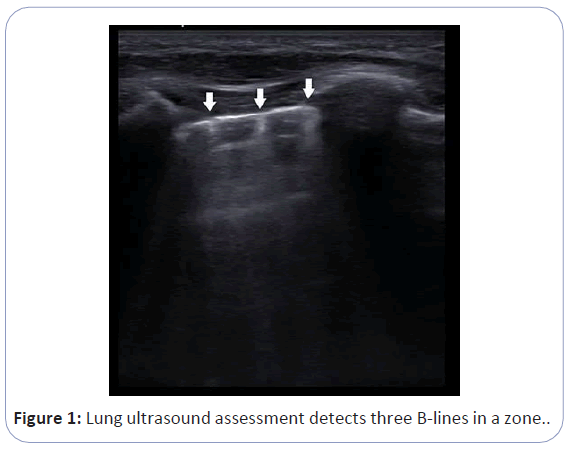
Lung Ultra Sound (LUS) and inferior vena cava collapsibility index (cIVC) measurement methods may assist the detection of fluid overload in hemodialytic patients. Lung ultrasound is generally used to evaluate acute cardiopulmonary respiratory failure, consolidation, pleural effusion, and pneumothorax. There have also been reports that POCUS may produce important findings in the evaluation and follow-up of the fluid status of the patients. A trained observer can easily and quickly assess the extravascular lung water with POCUS. LUS can detect artifacts known as B-lines in patients with fluid overload as shown in Figure 1. Heldeweg et al. reviewed the effect of LUS-guided management on cumulative fluid balance and other clinical outcomes [5]. They concluded that LUS-guided management, exclusively or in concert with other diagnostic modalities, has the potential to improve patient care. Although data are limited, adult studies show the efficiency of LUS in detecting dry weight and fluid overload in hemodialysis patients. Current information on this topic in pediatric literature is based on observational studies.
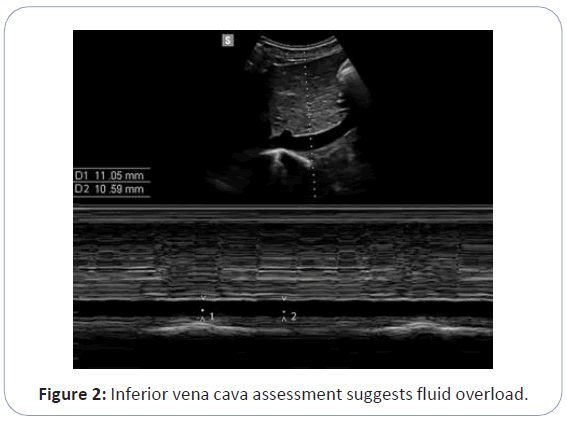
The Inferior Vena Cava (IVC) is a highly sensitive vessel to intravascular fluid status. IVC measurements using POCUS can suggest on the intravascular volume status; however, due to its elastic structure, extreme intravascular fluid variation may not correlate with variation in cIVC. cIVC have been used commonly to evaluate the fluid deficit of the patients. cIVC>40% have been considered to reveal fluid deficit in critically ill patients. However, there is not a threshold value that is widely accepted for fluid overload, and using the values that are determined for critically ill patients in patients on dialysis may not be appropriate. Figure 2 illustrates that inferior vena cava assessment suggests fluid overload in a patient [6,7].
Current information on the role of POCUS in regard of the assessment of predialytic fluid overload in hemodialytic children is scarce. In this article, we aim to review and emphasize the importance of a non-invasive bedside method of assessing the predialytic volume overload of children based on the published literature.
POCUS in Haemodialysis Units
Chronic volume overload has challenged nephrologists for decades, as it has been considered to be associated with poor outcomes. Chronic volume overload is common in patients on long-term hemodialysis, and can lead to heart failure and cardiac arrhythmias if left untreated. For this reason, fluid control is seen as a top priority in patients on dialysis [8,9].
The assessment of target weight and the management of fluid overload are still based on adult studies, as limited pediatric data are available. In various centers, fluid overload is generally determined using one or more of the following methods: physical examination, blood pressure, inter-dialytic weight gain, bioimpedance analysis, and relative blood volume monitoring. Each of these methods has advantages and disadvantages. Tools for assessment vary widely, but it is clear that none of them is adequate alone. Inter-dialytic weight gain, pre-dialytic blood pressure, and physical examination are currently preferred methods for fluid assessment in children on dialysis. However, current clinical assessments are not sufficient to optimize the target weight in children receiving dialysis [10]. This reinforces the importance of establishing useful, fast, and bedside methods
for determining volume status. A study, which investigates the application of artificial intelligence on dry weight and fluid overload determination, introduces a different perspective to the matter [11]. As this is still at a conceptual stage, further studies are required to assess the impact of the better dry weight control obtained with artificial intelligence. Large volumes of data from multiple inputs can augment the power of machine learning, and innovative studies that assess the capability of the ultrasonographic measurements on the prediction of fluid overload can present valuable inputs for machine learning systems.
Paediatric Literature on Lung Ultrasound
Although studies evaluating the role of LUS in children are increasing, the role of LUS in children on hemodialysis has not been studied extensively. Allinovi et al. first proposed the LUS as a novel technique for detecting fluid overload in children on dialysis [12]. They showed an improvement in B-line score correlated with reduction in fluid overload. Although it had several limitations, the work highlighted the value of LUS as a practical tool to aid optimization of the target weight in children on dialysis. Following this study, they then conducted a new study and also evaluated cIVC and bioimpedance spectroscopy (BIS) along with LUS [13]. The number of B-lines was the single parameter with the strongest linear correlation with fluid overload (r=0.57). They concluded that LUS might be superior to cIVC measurement and BIS in detecting volume overload in children with end-stage kidney disease (ESRD). Another recent study that reveals the value of LUS was conducted by Fu et al. [14]. They included 14 children with ESRD and assessed LUS in the interdialytic period and the dialytic period. In their study, the changes in the B-line scores were directly and positively correlated with interdialytic weight gain (r=0.517) and dialytic weight loss (r=0.558). They suggested that LUS could be a method to judge dry weight and volume change in children undergoing maintenance hemodialysis. In addition, we performed a study that combined LUS and cIVC to predict fluid overload in dialytic children [15]. There was a strong positive correlation between the predialysis total number of B-lines assessed by 12-site method and predialysis fluid overload (r=0.764). The total number of B-lines was found to successfully predict fluid overload (relative hydration >+7%) and severe fluid overload (relative hydration >+15%) (AUROCs of 0.82 and 0.80 respectively). We showed that having ≥ 10.5 predialysis total number of B-lines was associated with fluid overload with 77% sensitivity and 85% specificity.
All of these studies have one or more limitations and methodological differences between them. First and most importantly, there is no standardized examination method for LUS. The eight-site, 12-site, 14-site, or 28-site examination methods have been used to assess fluid overload in pediatric hemodialysis patients [12-16]. Different examination methods in a patient may cause different total B-line scores and statistical differences in general. This situation hinders an objective comparison on the studies. Other important issues that can hinder effective comparison are the small number the patients, heterogeneous patients’ diagnosis and dialysis modality, different age groups, the lack of an objectively determined target weight, and the use of different gold standard comparators to assess fluid overload. Notwithstanding these limitations and differences, existing data demonstrate clear potential of ultrasonography in determining fluid overload and optimizing target weight in children with ESKD. Further studies with standardized methods are potentially needed to show the reliability and validity of the POCUS.
Paediatric Literature on Inferior Vena Cava Collapsibility
Assessment of inferior vena cava diameter in children on hemodialysis was first reported in 1996 [17]. However, it was Hacıomeroglu et al. who first studied cIVC changes in children on dialysis or continuous ambulatory peritoneal dialysis and showed that cIVC is influenced by even minimal volume changes [18]. To date, although there are many studies that evaluate the cIVC and fluid responsiveness, very few studies have assessed the association of cIVC and fluid overload in children on hemodialysis [13-18]. Perhaps the most important reason for this is that the capability of IVC is limited to intravascular volume status. The fact is the vessels may dilate slightly, and excess fluid accumulation realizes, especially in extravascular compartments.
Discussion
The limitation of IVC measurement on the assessment of extravascular fluid volume reduces the reliability in children on hemodialysis. Secondly, change in cIVC may not correlate with the extreme variation in the fluid status of the patients. The difficulty in obtaining adequate images, and the unsuitability in patients who are unable to cooperate with holding their breath, are other limitations.
Hacıomeroglu et al. compared the change in cIVC before and after hemodialysis, and showed a significant increase in cIVC after haemodialysis as expected [18]. However, they did not measure the volume overload degree and did not search for the correlation with cIVC. In a small sample observational study, Allinovi et al. evaluate the accuracy of BIS, cIVC, and LUS in detecting fluid overload in children with ESRD [13]. As mentioned before, they concluded that LUS might be superior to cIVC measurement and BIS in detecting volume overload in children with ESRD. There was a non-significant negative linear relationship between fluid overload by weight and cIVC (r=-0.24). However, clinically, most of the patients (82%) were considered as euvolaemic, and 14% of the patients were dehydrated. Median fluid overload by weight for all assessments was 0.5% (range -5.0 to 6.0%). It is no surprise that they could not show correlation between cIVC and fluid overload with this patient population. In our study, there was a moderate negative correlation between the predialysis cIVC and predialysis fluid overload (r=-0.599). Furthermore, we showed that cIVC could successfully predict fluid overload and severe fluid overload (AUROCs 0.80 and 0.76, respectively). The best cutoff point for cIVC was ≤ 23.5 for overhydration, and ≤ 18.2 for severe over hydration. We found cIVC measurement, in addition to LUS, resulted in better fluid overload prediction. This study also has several limitations, as it is the first study that combined LUS and cIVC to predict fluid overload in dialytic children. Randomized controlled studies are needed to prove the reliability of the combined use of LUS and cIVC in the assessment of predialysis fluid overload.
Conclusion
The number of studies evaluating the reliability of POCUS in pediatric hemodialysis units has been increasing recently. However, current information on this regard is scarce. Well- designed studies with larger samples are needed to prove the reliability of the use of lung ultrasound and cIVC in the assessment of predialysis fluid overload in children.
Compliance with Ethical Standards
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have declared that they have no competing or potential conflicts of interest.
References
- Soni NJ, Schnobrich D, Mathews BK (2019) Point-of-care ultrasound for hospitalists: A position statement of the Society of Hospital Medicine. J Hosp Med 14: E1-E6.
- Soni NJ, Tierney DM, Jensen TP, Lucas BP (2017) Certification of point of-care ultrasound competency. J Hosp Med 12: 775-6.
- ACP statement in support of point of-care ultrasound in internal medicine. American College of Physicians. 2021
- Singh Y, Tissot C, Fraga MV (2020) International evidence-based guidelines on Point of Care Ultrasound(POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care 24: 65.
- Heldeweg MLA, Jagesar AR, Haaksma ME, Smit JM, Paulus F, et al. (2021) Effects of Lung Ultrasonography-Guided Management on Cumulative Fluid Balance and Other Clinical Outcomes: A Systematic Review. Ultrasound Med Biol 47: 1163-1171.
- Yildizdas D, Aslan N (2020) Ultrasonographic inferior vena cava collapsibility and distensibility indices for detecting the volume status of critically ill pediatric patients. J Ultrason 20: e205-e209.
- Muller L, Bobbia X, Toumi M, Louart G, Molinari N, et al. (2012) Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: Need for a cautious use. Crit Care 16: R188.
- Sinha AD, Agarwal R (2017) Setting the dry weight and its cardiovascular implications. Semin Dial 30: 481-8.
- Weiner DE, Brunelli SM, Hunt A, Schiller B, Glassock R, et al. (2014) Improving clinical outcomes among hemodialysis patients: a proposal for a “volume first” approach from the chief medical officers of US dialysis providers. Am J Kidney Dis 64: 685-95.
- Muller L, Bobbia X, Toumi M, Louart G, Molinari N, et al. (2012) Respiratory variations of inferior vena cava diameter to predict fluid responsiveness in spontaneously breathing patients with acute circulatory failure: Need for a cautious use. Crit Care 16: R188.
- Sinha AD, Agarwal R (2017) Setting the dry weight and its cardiovascular implications. Semin Dial 30: 481-8.
- Weiner DE, Brunelli SM, Hunt A, Schiller B, Glassock R, et al. (2014) Improving clinical outcomes among hemodialysis patients: a proposal for a “volume first” approach from the chief medical officers of US dialysis providers. Am J Kidney Dis 64: 685-95.
- Hayes W, Paglialonga F (2019) Assessment and management of fluid overload in children on dialysis. Pediatr Nephrol 34: 233-42.
- Fu Q, Chen Z, Fan J, Ling C, Wang X, et al. (2021) Lung ultrasound methods for assessing fluid volume change and monitoring dry weight in pediatric hemodialysis patients. Pediatr Nephrol 36: 969-76.
- Yontem A, Cagli C, Yildizdas D,Horoz O, Ekinci F et al. (2021) Bedside sonographic assessments for predicting predialysis fluid overload in children with end-stage kidney disease. Eur J Pediatr 180: 2017-2022
- Niel O, Bastard P, Boussard C, Kwon T, Hogan J, et al. (2018) Artificial intelligence outperforms experienced nephrologists to assess dry weight in pediatric patients on chronic hemodialysis. Pediatr Nephrol 33: 1799-803.
- Allinovi M, Saleem M, Romagnani P, Nazerian P, Hayes W (2017) Lung ultrasound: a novel technique for detecting fluid overload in children on dialysis. Nephrol Dial Transplant 32: 541-547.
- Allinovi M, Saleem MA, Burgess O, Armstrong C, Hayes W (2016) Finding covert fluid: methods for detecting volume overload in children on dialysis. Pediatr Nephrol 31:2327-2335.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences