The Use of Everolimus for Renal-Transplant Patients with Nephrotoxicity by Calcineurin Inhibitors in our Center: Case Report
Koichi Kozaki, Kenji Yuzawa and Haruo Ohtani
DOI10.21767/2472-5056.100027
Koichi Kozaki1,2* and Kenji Yuzawa2
1Department of Surgery, National Hospital Organization Mito Medical Center, Ibaraki, Japan
2Department of Transplantation Surgery, National Hospital Organization Mito Medical Center, Ibaraki, Japan
- *Corresponding Author:
- Koichi Kozaki
Department of Surgery
National Hospital Organization Mito Medical Center
Ibaraki, Japan
Tel: 81292407711
Fax: 81292407788
E-mail: k.kozaki.d@mn.hosp.go.jp
Received Date: January 20, 2017; Accepted Date: January 24, 2017; Published Date: January 27, 2017
Citation: Yuzawa K, Kozaki K (2017) The Use of Everolimus for Renal-Transplant Patients with Nephrotoxicity by Calcineurin Inhibitors in our Center: Case Report. J Clin Exp Nephrol 1:27. DOI: 10.21767/2472-5056.100027
Copyright: © 2017 Kozaki K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Long-term renal-transplantation outcomes are reportedly impaired by acute and chronic nephrotoxicity caused by calcineurin inhibitors (CNI), while recent renal transplant results have improved remarkably due to progress in the development of immunosuppressive drugs. Everolimus (EVL) belongs to a novel class of immunosuppressive agents, known as mammalian target of rapamycin (mTOR) inhibitor or proliferation signal inhibitor, which allow dose reduction or even complete withdrawal of CNI. mTOR inhibitor, such as EVL appear to exacerbate pre-existing proteinuria and may ameliorate nephrotoxicity caused by CNI. We describe herein one patient showing histological improvement of CNI nephrotoxicity with EVL.
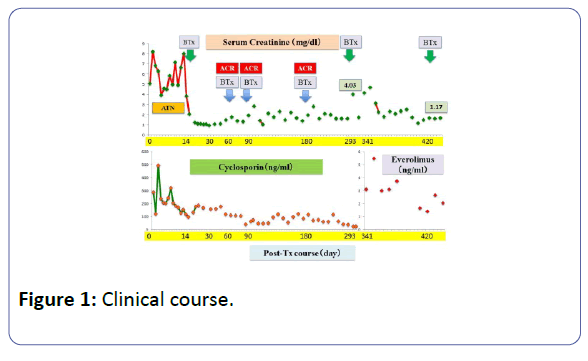
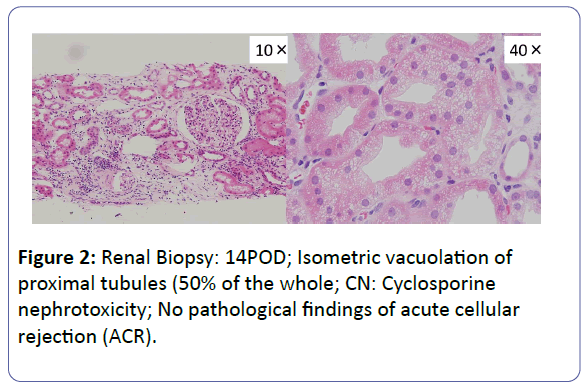
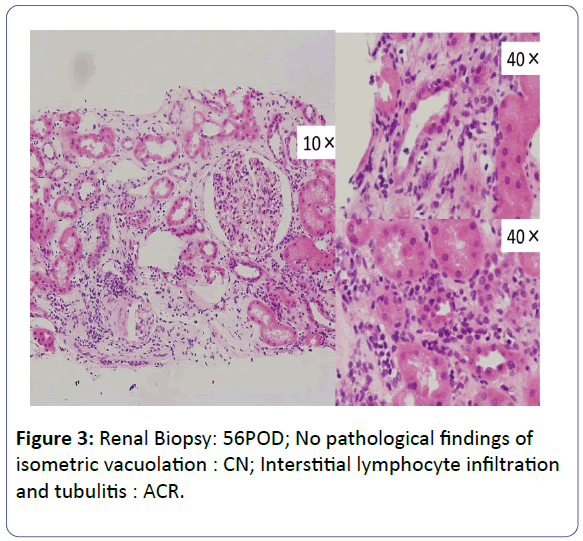
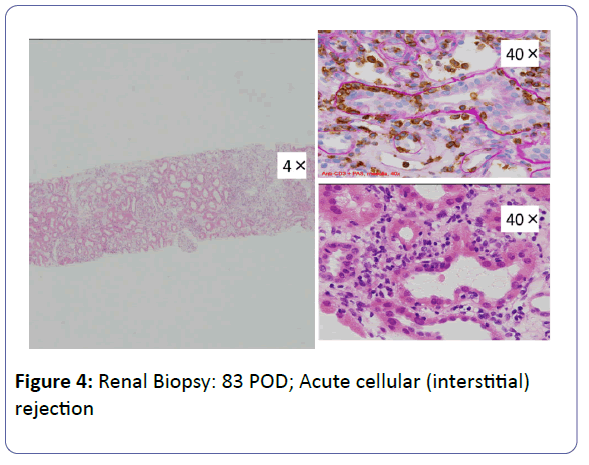
Patient: A 53-year-old, female underwent her third living-donor transplantation. We used cyclosporine (CsA), mycophenolate mofetil (MMF), prednisolone (PSL) and basiliximab. Diuresis was obtained immediately, but on post-operative day 3 (POD 3), urine volume decreased and renal function gradually deteriorated. On POD 14, renal biopsy (BTx) revealed CsA-induced nephrotoxicity. Renal function improved with a reduction in the CsA dose, and the patient was subsequently discharged on POD 30 with a serum creatinine level (S-Cr) 1.07 (mg/dL). Acute r rejection occurred three times, but each time renal function was improved by anti-rejection therapy, and subsequently stabilized with S-Cr levels in the 1.4-1.6 range. S-Cr suddenly rose to 4.03 on POD 293. Histopathological examination showed CsA nephrotoxicity. Although we reduced the CsA dose, her renal function remained unstable. We changed CsA to EVL on POD 341, and renal function subsequently stabilized. On POD 420, her S-Cr level was 1.17, and BTx showed amelioration of nephrotoxicity findings.
Conclusion: In this patient, we obtained improvement of renal dysfunction caused by CsA nephrotoxicity without rejection by switching CsA to EVL (EVL +MMF+PSL). EVL can be safely used as an alternative to CsA, and it is anticipated that EVL+MMF+PSL will become one of the choices for immunosuppressive therapy in the future.
Keywords
Immunosuppressive therapy; Nephrotoxicity; Renal transplantation
Introduction
With recent progress in immunosuppressive therapy, shortterm transplantation outcomes have improved remarkably. However, from a long-term viewpoint, a number of problems remain unsolved such as chronic impairment of the transplanted kidney, infections and malignant tumors arising during the management of post-transplant patients. Nephrotoxicity associated with calcineurin inhibitors (CNI) is a particularly challenging problem. As we think about dose reductions and withdrawal of CNI including cyclosporine (CsA), as a means of reducing the nephrotoxicity of CsA in the future, everolimus (EVL), an mTOR inhibitor, is expected to play an important role [1]. In our department, EVL has thus far been used for induction/ maintenance therapy in two cases, and for CsA nephrotoxicity in three others (Table 1). We report our experience with a case of CsA nephrotoxicity that developed after transplantation of a kidney in which renal function was improved by switching from CsA to EVL.
| Patient | Tx | Use reason for EVL | Immuno-suppression | Latest S-Cr (mg/dl) |
Adverse effect |
|---|---|---|---|---|---|
| 23y/M | Living | Induction/ maintenance |
CsA+EVL+ (PSL) | 1.24 | |
| 53y/F | 3rd Living | CAN | CsAÃÆâÃâÃ
¾Ãâââ¬ÂEVL EVL+MMF+PSL |
1.17 | Hyper-lipidemia |
| 34y/F | ABO-incompatible Living |
CAN | CsAÃÆâÃâÃ
¾Ãâââ¬ÂEVLÃÆâÃâÃ
¾Ãâââ¬ÂCsA CsA+EVL+MMF+PSL |
3.2 | Proteinuria |
| 62y/M | Caderver | CAN | CsAÃÆâÃâÃ
¾Ãâââ¬ÂEVL EVL+MMF+PSL |
2.5 | Urinary tract infection |
| 61y/F | Caderver | Induction/ maintenance |
CsA+EVL+ PSL | 0.97 | Herpes Zonster |
| CAN: Chronic Alogtaft Nephropathy; CsA: cyclosporine; EVL: Everolimus; MMF: Mycophenolate Mofetil; PSL: Prednisolone |
|||||
Table 1: Patients Everolimus Case.
Case Report
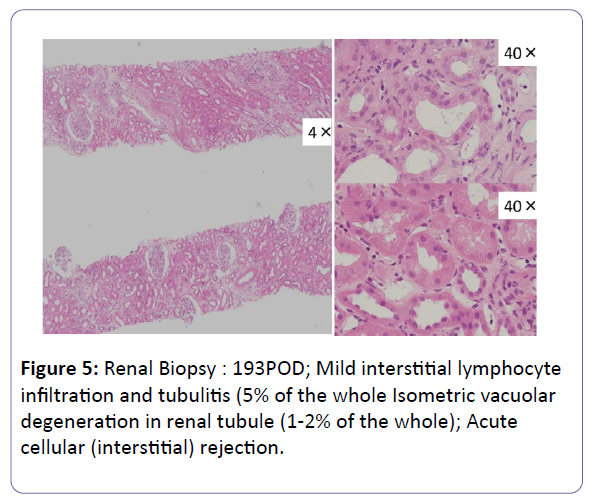
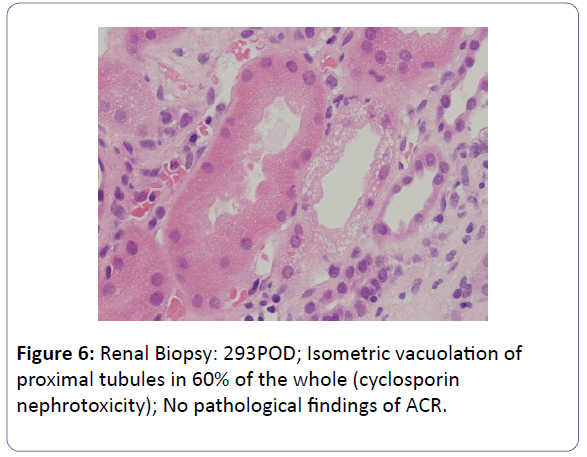
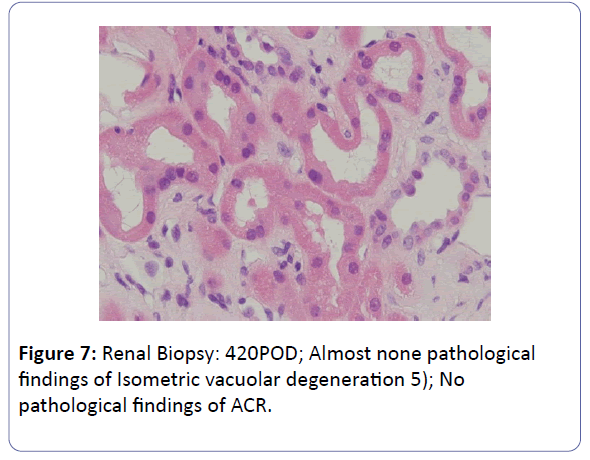
The patient was a 53-year-old woman. She had been suffering from chronic renal failure due to hypertension associated with pregnancy, and had already undergone living kidney transplantation twice. However, renal transplant function was impaired by chemotherapy for malignant lymphoma that developed after transplantation and she was thus maintained on dialysis. She then received living-donor kidney transplantation a third time with her 50-year-old husband serving as the donor. Because she was positive for anti-HLA antibody, she underwent preoperative desensitization therapy, and immunosuppression was induced and maintained with 4 agents, CsA, mycophenolate mofetil (MMF), prednisolone (PSL) and basiliximab. Renal function was immediately restored, with prompt diuresis, by the newly transplanted kidney. However, since urinary output began to gradually decrease on postoperative day 3 (POD 3), renal biopsy (BTx) was performed on POD 14, and CsA nephrotoxicity was diagnosed. The CsA dose was reduced, resulting in gradual improvement of renal transplant function and serum creatinine level (S-Cr) reached 1.07 (mg/dL) on POD 30. She was thus discharged. Subsequently, she developed chronic rejection (CR) twice, but rejection was prevented with anti-rejection therapy and she remained stable with S-Cr of 1.4-1.6 (mg/dL). BTx on that occasion showed a mixture of the components of CsA nephrotoxicity. On POD 293, S-Cr suddenly rose to 4.03 (mg/dL), necessitating BTx. Again, CsA nephrotoxicity was diagnosed. The CsA dose was reduced, but renal transplant function did not stabilize. Thus, CsA was replaced with EVL on POD 341, and EVL +MMF+PSL was established as maintenance immunosuppressive therapy. The initial EVL dose was 1.5 (mg/day), with a target trough level of 3-8 (ng/mL). At this dose of EVL, renal transplant function gradually improved, and S-Cr decreased to 1.17 (mg/dL) on POD 420. BTx also showed improvement of CsA nephrotoxicity (Figures 1-7).
Discussion
Chronic allograft nephropathy (CAN) / interstitial fibrosis and tubular atrophy (IFTA) is factor disturbing the long-term survival of the renal transplantation [2-5]. One of the factors of CAN is CNI nephropathy. Kreis et al. and Nankivell et al. reported that most renal transplant patients exhibit signs of CNI nephrotoxicity within 1 year of renal transplantation [6,7]. Moreover, Nankivell et al. reported that lowering CNI dose can reduce CNI nephrotoxicity [7]. Therefore, we should reduce a dose complete withdrawal of CNI as soon as possible, or change CNI to other drugs including EVL for preventing CNI nephropathy with transplant kidney.
EVL is an immunosuppressive agent that exerts a proliferation-inhibiting effect on T, B and vascular smooth muscle cells. It is characterized by the absence of nephrotoxicity such as that associated with CNI, but induces adverse reactions such as hyperlipidemia, bone marrow suppression, delayed wound healing and stomatitis. The lymphocyte incidence is reportedly increased if EVL use begins immediately after renal transplantation. Because EVL produces its effect by arresting the cell cycle, it is frequently used in place of a metabolic inhibitor [5,8]. In 2011, Budde et al. reported that they reduced the CNI dose to diminish CNI nephrotoxicity in renal transplantation cases and switched to EVL -based immunosuppressive therapy, which proved effective [9]. Since then, the effectiveness of EVL-based immunosuppressive therapy in reducing CNI nephrotoxicity has been reported by a number of institutions. In our hospital, EVL has been used on five cases. It was used for induction and maintenance of immunosuppression in two cases, and against CsA nephrotoxicity in three others, with the adverse reactions of hyperlipidemia, hypertension, proteinuria and infection. In two cases, one with severe proteinuria and the other with a serious infection, CsA replaced EVL, but renal function was good in the other three cases. In the present study, EVL improved CNI nephrotoxicity in cases with acute nephrotoxicity that developed immediately after transplantation [10].
Acute nephrotoxicity manifests when CsA selectively causes constriction of afferent arterioles to reduce renal blood flow [11]. Switching from CsA to EVL in such cases has been reported, by many institutions, to produce relatively good results. On the other hand, the pathogenesis of chronic nephrotoxicity is considered to involve the effect of intrarenal arteriole constriction, repeated and maintained over a long period, impairment of vascular endothelial cells and acceleration of interstitial fibrosis due to CsA. According to reports describing a switch from CsA to EVL for chronic CNI-induced nephrotoxicity in patients with transplanted kidneys for 10 years or longer, there were no noticeable effects. The above findings suggest replacing CsA with EVL, as a means of ameliorating CNI nephrotoxicity, to be effective for acute nephrotoxicity while producing no appreciable effects on chronic nephrotoxicity in which the pathophysiological changes have already been completed [12,13].
In the treatment of CsA nephrotoxicity, switching from CsA to EVL was histopathologically confirmed to improve CsA nephrotoxicity, EVL was safely used as a substitute for CsA, and EVL+MMF+PSL can now be considered to be among the effective immunosuppressive regimens.
References
- Fernandez A, Marcen R, Galeano C, Caldés, Amezquita J, et al. (2009) Complete switch to everolimus in long-term kidney transplantation: Evolution of renal function. Transplant Proc 41: 2345-2347.
- Kumar J, Julie BM, Sharma A, Halawa A (2016) Systematic review on role of mammalian target of rapamycin inhibitors as an alternative to calcineurin inhibitors in renal transplant: Challenges and window to excel. Exp Clin Transplant doi: 10.6002/ect.2016.0270.
- Lin M, Mittal S, Sahebjam F, Rana A, Sood GK (2016) Everolimus with early withdrawal or reduced-dose calcineurin-inhibitors improves renal function in liver transplant recipients: A systematic review and meta-analysis. Clin Transplant doi: 10.1111/ctr.12872.
- Bemelman FJ, Fijter JW, Kers J, Meyer C, Peters-Sengers H, et al. (2016) Early conversion to prednisolone/everolimus as an alternative weaning regimen associates with beneficial renal transplant histology and function: The randomized-conteollrd MECANO trial. Am J Transplantation doi: 10.1111/alt.14048.
- Campistol JM, de Fijter JW, Nashan B, Holdaas H, Vitko S, et al. (2011) Everolimus and long-term outcomes in renal transplantation. Transplantation 92: S3-25.
- Kreis HA, Ponticelli C (2001) Causes of late renal allograft loss: chronic allograft dysfunction, death, and other factors. Transplantation 71: SS5-9.
- Nankivell BJ, Borrows RJ, Fung CL, OConnell PJ, Chapman JR, et al. (2004) Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation 78: 557-565.
- Pascual J (2009) The use of everolimus in renal-transplant patients. Int J Nephro and Renovas Dis 2: 9-21.
- Budde K, Becker T, Arns W, Sommerer C, Reinke P, et al. (2011) Everolimus-based, calcineurin-inhibitor-free regimen in recipients of de-novo kidney transplants: an open-label, randomized, controlled trial. The Lancet 377: 837-847.
- Budde K, Lehner F, Sommerer C, Arns W, Reinke P, et al. (2012) Conversion from cyclosporine to everolimus at 4.5 months posttransplant: 3-year results from the randomized ZEUS study. Am J Transplant 12: 1528-1540.
- Kihm LP, Hinkel UP, Michael K, Sommerer C, Seckinger J, et al. (2009) Contrast enhanced sonography shows superior microvascular renal allograft perfusion in patients switched from cyclosporine A to everolimus. Transplantation 88: 261-265.
- Nankivell BJ, Borrows RJ, Fung CLS, OConnell PJ, Allen RDM, et al. (2003) The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326-2333.
- Sánchez Fructuoso AS, Ruiz San Millan JC, Calvo N, Rodrigo E, Moreno MA, et al. (2007) Evaluation of the efficacy and safely of the conversion from a calcineurin inhibitor to an everolimus-based therapy in maintenance renal transplant patients. Transplant Proc 39: 2148-2150.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences