New Insights into the Interaction between Increased Iron-Deposition in the Kidney and Vitamin D/Klotho Axis in Diabetic Nephropathy
Nakhoul Nakhoul, Dahan Inbal, Evgeny Farber, Nakhoul Farid
DOI10.21767/2472-5056.100022
Nakhoul Nakhoul1, Dahan Inbal1, Evgeny Farber2 and Nakhoul Farid1,2,3*
1Diabetic and Metabolism Lab, Bar-Ilan University, Bar-Ilan, Israel
2Nephrology and Hypertension Division, Bar-Ilan University, Bar-Ilan, Israel
3Baruch Padeh Poriya Medical Center, Faculty of Medicine in Galilee, Bar-Ilan University, Bar-Ilan, Israel
- *Corresponding Author:
- Nakhoul Farid
Nephrology and Hypertension Division, Baruch-Padeh Poriya Medical center
Faculty of Medicine in Galilee, Bar Ilan University, Ramat Gan, Israel
Tel: 9724-6652587
E-mail: fnakhoul@poria.health.gov.il
Received date: November 03, 2016; Accepted date: November 22, 2016; Published date: November 25, 2016
Citation: Nakhoul N, Inbal D, Farber E, Farid N (2016) New Insights into the Interaction between Increased Iron-Deposition in the Kidney and Vitamin D/ Klotho Axis in Diabetic Nephropathy. J Clin Exp Nephrol 1:22. doi: 10.21767/2472-5056.100022
Copyright: © 2016 Nakhoul N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Diabetic Nephropathy (DN), a chronic complication of diabetes, is characterized by glomerular hyper-trophy, albuminuria, decreased glomerular filtration rate, and renal fibrosis resulting in end stage renal disease. Diabetic Nephropathy is one of the major micro-vascular complications of long term diabetes mellitus. The pathogenesis of diabetic nephropathy is multifactorial. For many years it was consensus among scientists that hyper filtration and activation of the renin angiotensin aldosterone system is enough to develop kidney injury (diabetic nephropathy). The goal of this review is to describe new pathways involved in the pathogenesis of diabetic nephropathy. Recent studies showed that new pathways are involved. One of these pathways is the increased production of free radicals via oxidative stress due to increased iron deposition in the lysosomes of the proximal convolute tubules. The increased oxidative stress in the lysosomes of the proximal convolute tubules can down regulate Klotho Protein expression and the synthesis of active vitamin D. The decrease in Klotho, active vitamin D and his receptor can aggravate the progression of diabetic nephropathy. AGEs-induced increased oxidative stress, also activated PKC-induced increased production of cytokines, chemokine’s and different inflammatory and apoptotic signals.
Another pathway involved in the pathogenesis of diabetic nephropathy is the altered autophagy process via hyperglycaemia induced activation of the mTORC1 in this review we will concentrate on new data published recently on these pathways involved in the pathogenesis of diabetic nephropathy and new treatments proposed.
Keywords
Diabetes mellitus; Haptoglobin; Iron oxidative stress; Klotho vitamin D; Vitamin D receptor
Abbreviations
• DN=Diabetic Nephropathy
• RAASi=Renin-Angiotensin II-Aldosterone System inhibitors
• Hp=Haptoglobin
• PCT=Proximal Convolute Tubule
• SGL2i=Sodium-Glucose Transport Inhibitor
Introduction
Diabetic Nephropathy is one of the major micro-vascular complications of diabetes mellitus resulting in end-stage renal disease (ESRD) necessitating renal replacement therapy within 20 years of DM onset. More than 40% of patients with DM will develop diabetic nephropathy (DN) after 10 years of diabetes mellitus onset. The number of patients suffering from DM is increasing every year. The pathogenesis of DN is multifactorial including genetic and environmental factors [1,2]. Risk factors such as arterial hypertension and genetic factors are important in the development of DN.
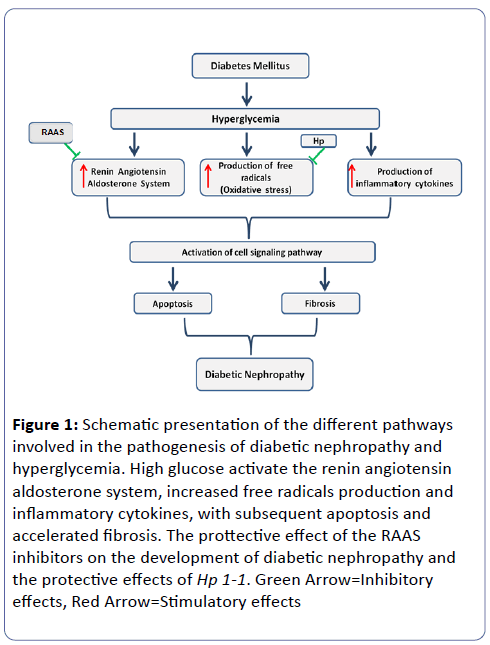
Its morphologic characteristics include glomerular hypertrophy, basement membrane thickening, mesangial expansion, tubular membrane hypertrophy, tubulo-interstitial fibrosis and arteriolar thickening and finally global glomerulosclerosis. Micro-albuminuria is an early sign of glomerular damage. Different pathways are involved in the pathogenesis of DN, and involved altered intracellular metabolism associated with hyperglycemia (Glucose toxicity), including the activation of protein kinase C, renin-angiotensin-aldosterone axis (RAAS) and the accumulation of advanced glycation end-products, accelerated oxidative stress, and altered apoptosis (Figure 1).
Figure 1: Schematic presentation of the different pathways involved in the pathogenesis of diabetic nephropathy and hyperglycemia. High glucose activate the renin angiotensin aldosterone system, increased free radicals production and inflammatory cytokines, with subsequent apoptosis and accelerated fibrosis. The prottective effect of the RAAS inhibitors on the development of diabetic nephropathy and the protective effects of Hp 1-1. Green Arrow=Inhibitory effects, Red Arrow=Stimulatory effects
Several lines of evidence have supported the concept that there exists polymorphic genetic loci which determine susceptibility to DN [3,4]. The importance of identifying a predictive marker would permit early identification of individuals at high risk of developing tubular and glomerular damage (Proximal and glomerular theory), in whom more aggressive blood glucose and blood pressure control might be initiated earlier, and may provide additional protection against the generation and progression of DN toward end stage renal disease. Understanding the new pathophysiology pathways of DN, and the development of new more specific therapies, such as new anti-diabetic drugs such as SGL2 inhibitor (SGL2i), and anti-oxidants such as vitamin E and iron chelating agent, can be of benefit in the future.
Early and chronic use of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers or a combination of both in DM patients, have limited renal protection, and can explain the increasing number of DM patients reaching the dialysis units or kidney transplantation [5]. Recently different pathways were suggested as mediators for hyperglycemia causing renal tissue damage. Between these mediators are the Haptoglobin (Hp) gene polymorphism, free iron release, oxidative stress, active vitamin D (1,25D3), Vitamin D receptor (VDR) and Klotho gene [6,7]. The presence or activation of autophagy was found to play a protective role in human and mice podocytes against high glucose-induced cell injury, which indicates a novel cellular mechanism and provides a potential therapeutic target for diabetic nephropathy, such as Metformine and SGL2i.
The Different Pathways
Haptoglobin (Hp)
Increased oxidative stress with free radicals, has been proposed to play a fundamental role in the development of DN [8,9]. Therefore, genetic loci encoding proteins regulating the level of oxidative stress are potential candidate susceptibility genes for DN. Different candidate genes have been identified in genetic association studies with DN [10]. One of these genes was Hp, a serum antioxidant protein which serves to protect against oxidative stress induced by extracorpuscular hemoglobin and iron release [11]. As reactive oxygen species, particularly those derived from iron, have been implicated in the progression of DN and other vascular complications of Diabetes, polymorphic genetic loci encoding variants in enzymes protecting against iron-induced oxidative stress serve as potential susceptibility determinants for the development of DN.
There are two common alleles at the Hp locus, denoted 1 and 2. The structure and function of the two Hp allele protein products are distinct. We and others have shown in vitro and in vivo that the Hp 1 protein is a superior antioxidant to the Hp 2 protein [12,13].
The two Hp alleles are in a balanced polymorphism with 40% of the alleles being Hp 1 and 60% Hp 2. In Israel the distribution of Hp 1-1 is (16%), Hp 2-1 (48%), and Hp 2-2 (36%) [14], in diabetic and non-diabetic individuals. We examined the association of DN and the Hp polymorphism and found that the Hp 1-1 genotype was associated with a significantly lower prevalence of DN [15,16] and an apparent slower rate of progression of DN to ESRD [17,18] compared with individuals with Hp 2-1 and Hp 2-2.
The Hp 2 allele is found only in humans [19]. All other animals (mice) have only the Hp 1 allele and therefore the Hp 1-1 genotype. One approach to model the Hp polymorphism in mice is to introduce the human Hp 2 allele as a transgene by producing a transgenic mouse with a genetically engineered murine Hp 2 allele and targeted insertion of this murine Hp 2 gene to the murine Hp locus by homologous recombination [20-22].
To study the importance of Hp genotype on the incidence and progression of diabetic nephropathy, we used diabetic mice with different Hp genotype (Hp 1-1, Hp 2-2, Hp 2-1). These mice become type 1 diabetes mellitus (DM) by using intraperitoneal Streptozotocin (STZ) administration at 6 weeks of age in a lowdose 5-day protocol, approved by the NIH (50 mg/kg for 5 days), were used to study the mechanisms of the different proposed pathways. We published different studies in human and mice showing the better protective effects of Hp 1-1 genotype against the development of vascular complications of DM.
Iron and oxidative stress
Iron plays an important role in maintaining physiological homeostasis in the body. However, excess iron can lead to free radical damage via the Fenton reaction, resulting in cell damage. Reactive oxygen species (ROS), particularly those derived from excessive labile iron, have been implicated in the increase of oxidative stress injury in the renal proximal convolute tubular cells (PCT), and podocytes with progression of DN and other vascular complications of DM [23,24]. The persistent state of hyperglycemia affects the renin-angiotensin system and the signaling of the transforming factor (TGF-β) with consequent chronic inflammation and sclerosis.
The major function of the Hp protein is to bind and modulate the fate of extra-corpuscular hemoglobin and its iron load. This complex of Hb-Hp is cleared from the circulation by the receptor CD 163 on the macrophage. It is important to mention that the efficiency of the Hp 1-1 to clear the free iron from the circulation via the CD 163 receptor, is more efficient than Hp-2-2.
We have previously demonstrated an interaction between the Hp genotype and DM on the increased accumulation of iron in the lysosomes of the renal PCT cells [25,26]. As we mentioned before, in Hp 2-2 there is massive glomerular filtration of iron in the capillary wall, with increased reabsorption and lysosomal deposition, in the PCT cells.
We used special transmission electron microscopy, (TEM) and and electron energy loss spectroscopy (EELS) to demonstrated the marked accumulation of electron-dense deposits in the lysosomes of proximal tubules cells in Hp 2-2 DM mice [25]. These deposits were iron rich, and are associated with lysosomal membrane lipid peroxidation and loss of lysosomal membrane integrity with consequent decrease in different proteins expression. Klotho and vitamin D and his receptor are part of these proteins.
Kan Saito et al. have shown that iron overload decreased renal expression of the klotho gene at both the mRNA and the protein level, and that iron chelation can upregulate klotho expression [27]. Their findings suggest that, the mechanisms of downregulation of klotho may be mediated by abnormal iron metabolism in the kidney elicited by angiotensin II [28].
Iron-induced renal tubular injury via oxidative stress and apoptosis, and altered klotho level, may play a major role in the development of DN, and may be a future therapeutic target such as with chelating agents such as Deferiprone and vitamin E, for slowing the progression of DN [29].
Klotho is a novel anti-aging gene encoding a protein with a multiple pleiotropic effects. αKlotho gene is composed of five exons, in humans and mice, it’s highly expressed in the distal and proximal convolute tubular epithelium of normal kidneys. The soluble α klotho is characterized by his anti-apoptotic effects especially in DN. Several studies have shown that α klotho expression is decraesed in the renal PCT cells in early stages of DN, in humans and mice model [30,31]
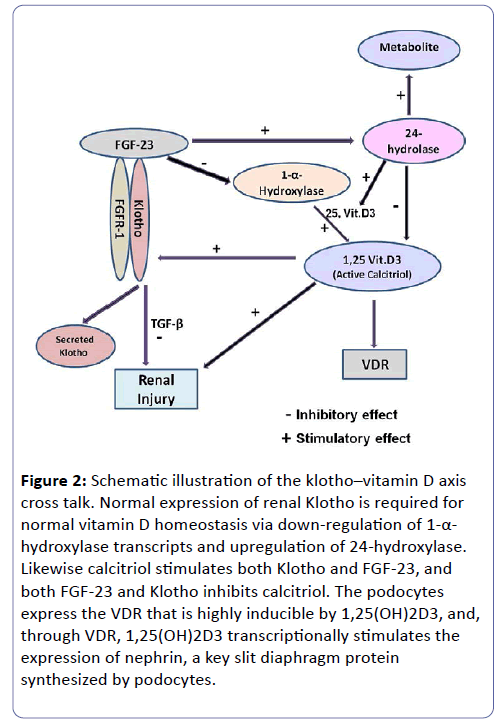
Interesting cross talk exist between α klotho-Vitamin D/ Vitamin D receptor- (VDR) axis. Up regulation or restoration of klotho by 1,25(OH)2D3, may provide a mean to slow down the progression of chronic diabetic kidney disease (CDKD). Klotho slows the renal deterioration and the cardio-vascular complications via different inhibitory effects on Transforming Growth Factor β (TGF-β), and suppressive effects on reactive oxygen species production (ROS). klotho also exerts inhibitory action on renal fibrosis and cardio-vascular atherosclerosis in high oxidative stress conditions such as hyperglycemia and DM (Figure 2) [30-35]. Our unpublished results show that diabetic Hp 2-2 mice and humans have reduced expression of klotho in the PCT cells in early and late stages of DM vs Hp-1-1.
Figure 2: Schematic illustration of the klotho–vitamin D axis cross talk. Normal expression of renal Klotho is required for normal vitamin D homeostasis via down-regulation of 1-α- hydroxylase transcripts and upregulation of 24-hydroxylase. Likewise calcitriol stimulates both Klotho and FGF-23, and both FGF-23 and Klotho inhibits calcitriol. The podocytes express the VDR that is highly inducible by 1,25(OH)2D3, and, through VDR, 1,25(OH)2D3 transcriptionally stimulates the expression of nephrin, a key slit diaphragm protein synthesized by podocytes.
Vitamin D/VDR signaling
Vitamin D is biologically inactive and requires enzymatic conversion to active metabolites. Vitamin D is transported to the liver, where it is first hydroxylated to yield 25-hydroxyvitamin D. Then 25-Hydroxyvitamin D is further hydroxylated by 1-α- hydroxylase in the PCT of the kidney, to the active form 1,25- dihyroxyvitamin D [36-38]. The active 1,25-dihydroxyvitamin D binds to the intracellular vitamin D receptor (VDR) to activate vitamin D response elements within target genes [39]. In the kidney, vitamin D is important for maintaining podocytes integrity, and suppressing renin gene expression and inflammation [39,40]. 1,25- dihydroxyvitamin D progressively decrease due to PCT injury by iron and ROS production, leading to a vitamin D deficient state. The Vitamin D analogues supplementation as Calcitriol or Paricalcitol (19-nor-1,25- dihydroxyvitamin D2), improves the renal damage of diabetic kidney disease patients and their survival (Figure 2) [41,42].
Increasing prevalence of DN and ESRD in spite of massive RAAS inhibition (ACEI/ARBs), has made the need for new effective treatment of DN. Thereby identifying new therapeutic targets beyond RAAS inhibitors is required in order to improve clinical management. Recently Wang et al. from Chicago [41] showed that vitamin D/VDR signaling in podocytes plays a critical role in the kidney protection from diabetic injury and reconstitution of VDR null mice with the human VDR (hVDR) transgene in podocytes rescued the severe diabetes-related renal damage. Experimental studies showed that administration of a selective vitamin D analogue like Paricalcitol reduces albuminuria [42].
Furthermore, recent data suggest that Paricalcitol, added to renin angiotensin aldosterone system (RAAS) blockade, further reduces albuminuria in people with Type 2 diabetes and diabetic nephropathy. The Renin inhibitor (Aliskiren) plus Paricalcitol, significantly decreased interstitial fibrosis volume when compared to monotherapy [43,44].
Autophagy
Autopahagy is a highly regulated lysosomal protein degradation pathway that removes protein aggregates and damaged or excess organelles in order to maintain intracellular homeostasis and cell integrity. Recently a review by Mary Choi and her group [42] showed that the accumulation of damaged proteins and organelles is associated with the pathogenesis of DN. Autophagy pathway in the kidney is activated under high glucose conditions, with increased oxidative stress via free radicals in PCT cells, and in podocytes. The formation of autophagosomes depends on several genes including Beclin-1, and other autophagy-related (Atg) genes, especially Atg-5. Genetic evidence have led to the hypothesis that autophagy is involved in the pathogenesis and progression of DN (i.e., polymorphisms in ATGs).
The autophagy-lysosomal degradation pathway is likely to play an essential role in maintaining podocyte function and integrity. Podocytes exhibit active autophagy process under normal glucose levels, suggesting that podocytes require a high basal level of autophagy to maintain podocytes and glomerular maintenance [42-45]. Furthermore, it is interesting that the loss of autophagy in podocytes affects the ultrastructure and function of these cells but also that of nearby mesangial cells, which become sclerotic.
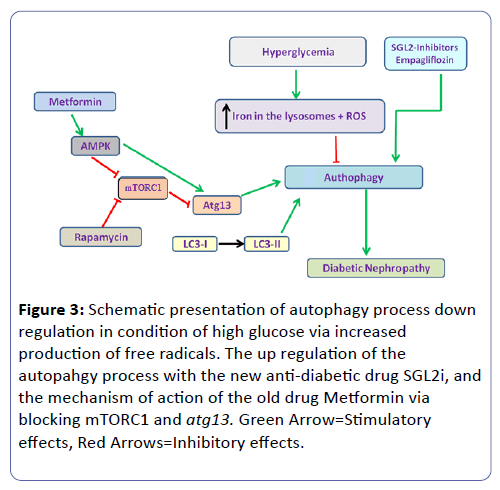
New evidence suggests that targeting the autophagic pathway to activate and restore autophagy activity may be Reno-protective in diabetic patients, especially via the mTORC1 (Figure 3) [45].
Figure 3: Schematic presentation of autophagy process down regulation in condition of high glucose via increased production of free radicals. The up regulation of the autopahgy process with the new anti-diabetic drug SGL2i, and the mechanism of action of the old drug Metformin via blocking mTORC1 and atg13. Green Arrow=Stimulatory effects, Red Arrows=Inhibitory effects.
Discussion
In humans, hyperglycemia has been shown to be necessary, but not sufficient, to induce the development of micro- and macrovascular complications. Genetic differences between diabetic patients are important in the determination of why some patients develop these complications and others do not. To investigate and treat diabetic patients in early reversible stages, we used our diabetic mice model. As we have shown in an earlier publication [26], the typical structural changes of DN, were increased iron deposition in the lysosome of the renal PCT cells, which can increase the generation of reactive oxygen species and cell damage [26].
Hp 2-2 DM in humans and mice has more renal damage compared with the protective Hp 1-1 DM [16]. This can be explained by differences in the manner in which the two Hp types regulate the disposition of extra corpuscular hemoglobin and more specifically hemoglobin-derived iron [3,4]. Hp 1-1 protein is superior to the Hp 2-2 protein in binding to this free hemoglobin and neutralizing its oxidative potential. Furthermore, the rate of clearance of hemoglobin is Hp type dependent. Hp 1-1 directs a more rapid clearance of free hemoglobin via uptake by the CD163 macrophage scavenger receptor. In the diabetic state, this is particularly important as the ability of Hp to block the oxidative activity of hemoglobin is impaired when hemoglobin becomes glycated [12,13]. Patients and mice with the Hp 2-2 genotype, the clearance of iron is impaired and are prone to microvascular complications (Nephropathy and Retinopathy) with increased mortality [13-15]. Iron chelation by oral Ferriprox (Deferiprone) has a renoprotective effect in DN rats by relieving oxidative stress, inflammation, and fibrosis. Further, hyperglycemia increases both iron accumulation and cell senescence in PCT cells, and macrophage infiltration in the kidney of STZ-induced type 1 diabetic mice. The inhibition of dietary iron absorption by Desferroxamine (DFX) suppressed the increase in proximal tubular iron accumulation and macrophage infiltration into the interstitial space.
Due to the increase in number of patients with type 2 DM with diabetic nephropathy reaching the hemodialysis unit, in spite of chronic and maximal dose of ACEI and ARBs, blocking the RAAS, other agents such as anti-oxidant and iron chelators were investigated. Between these is vitamin E supplementation to DM type 2 patients. Our results had demonstrated that vitamin E appears to provide renal protection to Hp 2-2 DM mice but does not appear to have any effect on Hp 1-1 DM mice [45,46]. The pharmacogenomic implications of these findings are significant. Clinical Studies assessing the effect of vitamin E on the progression of DN in humans with DM are not conclusive. Moreover, recent meta-analysis suggested that there is an increased risk of all cause mortality with high-dose vitamin E supplementation. The ability of vitamin E to reduce features of renal disease characteristic of early human DN in Hp 2-2 DM mice but not in Hp 1-1 DM mice suggests that there may also be an interaction between Hp genotype and vitamin E therapy on diabetic renal disease [25,26]. We had published that the concentration of vitamin E in the lysosomes purified from Hp 2-2 DM mice kidneys was significantly lower than in lysosomes of Hp 1-1 [25].
Currently we are studying the consequences of increased iron deposition in our mice model with Hp 2-2 on the vitamin Dvitamin D Receptor-klotho axis. Our preliminary results showed a decrease in the α-1 hydroxylase, vitamin D receptor expression and increased apoptosis. All these alterations can be mediators in the development and progression of DN [1,2].
It is generally accepted that klotho expression in the kidney is markedly decreased in the early stages of DN in mice and humans [47-49]. Osamu Asai and his group [49], had published a paper on the role of klotho in special DN mice. In this study, they showed that renal Klotho expression levels were decreased in patients with early DN and in the streptozotocin (STZ) induced mouse model of T1D. Other study by Lee and his group [50] were the first to demonstrate that plasma and urine levels of soluble Klotho are significantly elevated in the DM patients compared to control subjects. Hence, klotho expression levels were decreased in kidneys of patients with early DN but with increased levels of plasma and urinary klotho. Although the exact mechanism of how klotho is reduced in diabetic kidney disease is not well understood, but the increased oxidative stress, and Ag-II may be involved [27,28]. As we mentioned before the increased iron deposition in the lysosomes of PCT cells, via Ag-II, can down regulate Klotho expression in the PCT. Accordingly, soluble Klotho may serve as a biomarker as well as a pathogenic factor for the progression of DN [28].
The role of active Vitamin D in the treatment of early stages of DN is not well understood or proven. The activities of 1,25 (OH)2D3 are mediated by the VDR, a member of the nuclear receptor super family. The so-called non-calcemic activities include regulation of renal and cardiovascular functions [36,40]. Relevant examples of these non-calcemic activities are the regulation of the RAAS in the kidney [38,39]. This includes the maintenance of podocytes integrity, suppressing renin gene expression and inflammation. Therefore, vitamin D has the potential to have a favorable impact in DN via VDR in kidney protection from DM oxidative stress injury [40,41]. Li and his group from Chicago [51] showed that Paricalcitol, the selective vitamin D receptor, has a podocyte protection and integrity in DN mice. The synergistic therapeutic effects of combined vitamin D analog Paricalcitol (19-nor-1,25-dihydroxyvitamin D2) with AT1 receptor antagonist (Losartan) on kidney disease in a model of type 2 diabetic mice, showed a dramatic therapeutic synergism, manifested by prevention of progressive albuminuria, restoration of the glomerular filtration barrier, reversal of the decline in slit diaphragm proteins, and reduction of glomerulosclerosis [43,52]. The expression of VDR in kidney tissue was significantly decreased in early stages of DN, and the down-regulation of VDR could be restored to the normal level by the treatment with Paricalcitol. Furthermore, the beneficial effects of Paricalcitol on renal fibrosis in DN is mediated by VDR via restoration of klotho expression [53]. Other studies had shown contoversial results on the effect of vitamin D on the kidney and heart. A review from Italy by Ciccone et al, discuss that hypovitaminosis D is associated with increased mortality from stroke and heart failure, and that administration of therapeutic vitamin D can increase mortality. Their conclusion was that the the role of vitamin D supplements in improving cardiovascular outcome in patients with diabetes with hypovitaminosis D remains to be determined [54].
Several studies published recently on autophagy as a new pathway involved in the pathogenesis of DN. The autophagy system is more than housekeeper system. The autophagy process is altered in both podocytes and PCT cells under high glucose condition and DM [55]. Autophagy can be induced by high glucose levels in various cell types including the podocytes, partly through hyperglycemia-mediated production of reactive oxygen species, and it has protective effects in vitro. In podocytes, high glucose levels lead to podocyte apoptosis in vitro that is mediated through caspase-3 activation.
Interestingly, in podocytes of diabetic mice and patients, mTORC1 is highly activated and may be involved in the mechanisms of diabetes-related autophagy inhibition in podocytes. These results suggest that the mTORC1-autophagy axis may be a future therapeutic target in diabetic nephropathy. The therapeutic potential of autophagy in DN, by the regulators of autophagy, especially via the mammalian target of Rapamycin (mTOR) complex 1 (mTORC1) [56]. Rapamycin, a potent mTORC1 inhibitor, can ameliorate glomerular lesions in diabetic animal models.
The acute exposure to high glucose-induced autophagy, which is mediated through the ANG-II with increased generation of ROS in podocytes. Recent papers published had shown that new anti-diabetic drugs such as, Dapagliflozin and others contain a potent selective reversible inhibitor of SGLT2, expressed exclusively in the proximal tubule of the kidney [57-59]. Between the new anti-diabetic medications that target a specific pathophysiologic pathway, the SGLT2 inhibitors and Metformin are of major interest in the future, in the treatment of early stage DN. Since SGLT2 inhibitors act via a different mechanism of action than metformin, the combination therapy of dapagliflozin and Metformin, is beneficial to treat T2DM and DN [59].
Conclusion
lysosomal iron overload decreased renal expression of klotho at both the mRNA and protein level, and that low iron diet and iron chelation drugs suppressed the angiotensin II-induced down regulation of this gene [59,60]. Furthermore, a free radical scavenger also suppressed the angiotensin II-induced downregulation of klotho, supporting the involvement of an increased production of reactive oxygen species in this process. This cross-talk between the free iron and klotho-Vitamin D-VDR axis is of great importance in understanding and treating our DN patients in the future.
References
- Mora-Fernández C, Domínguez-Pimentel V, de Fuentes MM, Górriz JL, Martínez-Castelao A, et al. (2014) Diabetic kidney disease: from physiology to therapeutics. J Physiol 592: 3997-4012.
- Chowdhury TA, Kumar S, Barnett AH, Bain SC (1995) Nephropathy in type 1 diabetes: the role of genetic factors. Diabet Med 12: 1059-1067.
- Conway BR, Savage DA, Brady HR, Maxwell AP (2007) Association between haptoglobin gene variants and diabetic nephropathy: haptoglobin polymorphism in nephropathy susceptibility. Nephron Exp Nephrol 105: e75-79.
- Imperatore G, Hanson RL, Pettitt DJ, Kobes S, Bennett PH, et al. (1998) Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Pima Diabetes Genes Group. Diabetes 47: 821-830.
- Mauer M, Zinman B, Gardiner R, Drummond KN, Suissa S, et al. (2002) ACE-I and ARBs in early diabetic nephropathy. J Renin Angiotensin Aldosterone Syst 3: 262-269.
- Huang JS, Chuang CT, Liu MH, Lin SH, Guh JY, et al. (2014) Klotho attenuates high glucose-induced fibronectin and cell hypertrophy via the ERK1/2-p38 kinase signaling pathway in renal interstitial fibroblasts. Mol Cell Endocrinol 390: 45-53.
- Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, et al. (200) Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404: 787–790.
- Suzuki D, Miyata T, Saotome N, Horie K, Inagi R, et al. (1999) Immuno-histo Chem ical evidence for an increased oxidative stress and carbonyl modification of proteins in diabetic glomerular lesions. J Am Soc Nephrol 10: 822–832.
- Levy AP (2003) Complication-resistant patients. In: Perspectives and Advances in Diagnosis and Treatment, edited by Shafrir E and Raz I. London: Martin Dunitz 55–68.
- Asleh R, Marsh S, Shiltruck M, Binah O, Guetta J (2003) Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res 92: 1193–1200.
- Asleh R, Guetta J, Kalet-Litman S, Miller-Lotan R, Levy AP (2005) Haptoglobin genotype- and diabetes-dependent differences in iron-mediated oxidative stress in vitro and in vivo. Circ Res 96: 435-441.
- Awadallah S, Hamad M (2000) The prevalence of type II diabetes mellitus is haptoglobin phenotype-independent. Cytobios 101: 145-150.
- Levy AP, Roguin A, Hochberg I, Herer P, Marsh S, et al. (2000) Haptoglobin phenotype and vascular complications in patients with diabetes. N Engl J Med 343: 969-970.
- Nakhoul FM, Zoabi R, Kanter Y, Zoabi M, Skorecki K, et al. (2001) Haptoglobin phenotype and diabetic nephropathy. Diabetologia 44: 602-604.
- Burbea Z, Nakhoul F, Rosenberg S, Zoabi R, Skorecki K, et al. (2004) Role of haptoglobin phenotype in end-stage kidney disease. Nephron Exp Nephrol 97: e71-76.
- Costacou T, Ferrell RE, Ellis D, Orchard TJ (2009) Haptoglobin genotype and renal function decline in type 1 diabetes. Diabetes 58: 2904-2909.
- Bowman BH, Kurosky A (1982) Haptoglobin: the evolutionary product of duplication, unequal crossing over, and point mutation. Adv Hum Genet 12: 189-261, 453-4.
- Miller-Lotan R, Herskowitz Y, Kalet-Litman S, Nakhoul F, Aronson D, et al. (2005) Increased renal hypertrophy in diabetic mice genetically modified at the haptoglobin locus. Diabetes Metab Res Rev 21: 332–337.
- Miller-Lotan R, Miller B, Aronson D, Asaf R, Nakhoul F,et al. (2007)Retinal capillary basement membrane thickness in diabetic mice genetically modified at the haptoglobin locus. Diabetes Metab Res Rev 23: 152–156.
- Levy AP, Levy JE, Kalet-Litman, Miller-Lotan R, Levy NS, et al. (2007)Haptoglobin genotype is a determinant of iron, lipid peroxidation and macrophage accumulation in the atherosclerotic plaque.ArteriosclerThrombVasc Biol 27: 134–140.
- Levy AP, Asleh R, Blum S, Levy NS, Miller-Lotan R, et al. (2010) Haptoglobin: basic and clinical aspects. Antioxid Redox Signal 12: 293-304.
- Melamed-Frank M, Lache O, Enav BI, Szafranek T, Levy NS, et al. (2001) Structure-function analysis of the antioxidant properties of haptoglobin. Blood 98: 3693–3698.
- Asleh R, Nakhoul F, Miller-Lotan R, Awad H, Farbstein D, et al. (2012) Poor lysosomal membrane integrity in proximal tubule cells of haptoglobin 2-2 genotype mice with diabetes mellitus. Free Radic Biol Med 53: 779-786.
- Nakhoul F, Nakhoul N, Asleh R, Miller-Lotan R, Levy AP (2013) Is the Hp 2-2 diabetic mouse model a good model to study diabetic nephropathy?Diabetes Res ClinPract 100: 289-297.
- Saito K, Ishizaka N, Mitani H, Ohno M, Nagai R (2003) Iron chelation and a free radical scavenger suppress angiotensin II-induced downregulation of klotho, an anti-aging gene, in rat,FEBS Letters 551: 58-62.
- Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, et al. (2005) Oxidative Stress Decreases Klotho Expression in a Mouse Kidney Cell Line. Nephron Exp Nephrol 101:e67–e74.
- Ishizaka N, Aizawa T, Yamazaki I, Usui S, Mori I, et al. (2002) Abnormal iron deposition in renal cells in the rat with chronic angiotensin II administration.Lab Invest 82: 87-96.
- Hu MC, Kuro-o M, Moe OW (2013) Renal and extrarenal actions of Klotho. Semin Nephrol 33: 118-129.
- Dërmaku-Sopjani M, Kolgeci S, Abazi S, Sopjani M (2013) Significance of the Anti-aging protein Klotho. MolMembr Biol 30: 369-385.
- Chang Hu M, Kuro-o M, Moe OW (2013) Renal and Extrarenal Actions of Klotho.Semin Nephrol 33:118-129.
- Schmid C, Neidert MC, Tschopp O, Sze L, Bernays RL (2013) Growth hormone and Klotho.J Endocrinol 219: R37-57.
- Hu MC, Kuro-o M, Moe OW (2012) The emerging role of Klotho in clinical Nephrology . Nephrol Dial Transplant 27: 2650-2657.
- Kacso IM, Bondor CI, Kacso G (2012) Soluble serum Klotho in diabetic nephropathy: relationship to VEGF-A. Clin Bio Chem 45: 1415-1420.
- Tangpricha V, Judd SE, Kamen D, Li YC, Malabanan A (2010) Vitamin d.Int J Endocrinol2010: 631052.
- Peng Y, Li LJ (2015) Serum 25-hydroxyvitamin D level and diabetic nephropathy in patients with type 2 diabetes mellitus.IntUrol Nephrol 47: 983-989.
- Li YC (2014) Discovery of vitamin D hormone as a negative regulator of the renin-angiotensin system. Clin Chem 60: 561-562.
- Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, et al. (2013) Molecular mechanisms of vitamin D action.Calcif Tissue Int 92: 77-98.
- Li YC (2013) Vitamin D receptor signaling in renal and cardiovascular protection. Semin Nephrol 33: 433-447.
- Wang Y, Deb DK, Zhang Z, Sun T, Liu W, et al. (2012) Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J Am Soc Nephrol 23: 1977-1986.
- De Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, et al. (2010) Selective vitamin D recepor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial.Lancet 376: 1543–1551.
- Deb DK, Sun T, Wong KE, Zhang Z, Ning G, et al. (2010) Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes.Kidney International 77: 1000–1009.
- Eren Z, Günal MY, Bakir EA, Coban J, ÇaÃÆââ¬Å¾Ãâà ¸layan B, et al. (2014) Effects of Paricalcitol and Aliskiren Combination Therapy on Experimental Diabetic Nephropathy Model in Rats.Kidney Blood Press Res 39: 581-590.
- Nakhoul FM, Miller-Lotan R, Awad H, Asleh R, Jad K, et al. (2009)Pharmacogenomic effect of vitamin E on kidney structure and function in transgenic mice with the haptoglobin 2-2 genotype and diabetes mellitus. Am J Physiol Renal Physiol 296: F830-838.
- Miller ER, Barriuso RP, Dalal D, Riemersma RA, Appel LJ, et al. (2005) Meta-analysis: high dosage vitamin E supplementation may increase all cause mortality. Ann Intern Med 142: 37–46.
- Xu Y, Sun Z (2015) (Molecular basis of Klotho: from gene to function in aging. Endocr Rev 36: 174-193.
- Guh JY, Chuang LY (2014) Klotho attenuates high glucose-induced fibronectin and cell hypertrophy via the ERK1/2-p38 kinase signaling pathway in renal interstitial fibroblasts.Molecular and Cellular Endocrinology 390: 45–53.
- Asai O, Nakatani K, Tanaka T, Sakan H, Imura A, et al. (2012) Decreased renal α-Klotho expression in early diabetic nephropathy in humans and mice and its possible role in urinary calcium excretion. Kidney Int 81: 539-547.
- Lee EY, Kim SS, Lee JS, Kim IJ, Song SH, et al. (2014) Soluble α-klotho as a novel biomarker in the early stage of nephropathy in patients with type 2 diabetes. PLoS One 9: e102984.
- Li YC, Kong J, Wei M, Chen ZF, Liu SQ, et al. (2002) 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229-238.
- Li YC (2003) Vitamin D regulation of the renin-angiotensin system.J Cell Bio Chem 88: 327-331.
- Ritter CS, Zhang S, Delmez J, Finch JL, Slatopolsky E (2015) Differential expression and regulation of Klotho by paricalcitol in the kidney, parathyroid, and aorta of uremic rats.Kidney Int 87: 1141-1152.
- Ciccone MM, Zito A, Dentamaro I, Vestito D, Scicchitano P, et al. (2015) Deficit di vitamina D e malattiecardiovascolari.G ItalCardiol 16: 16-20.
- Ding Y, Choi ME (2015) Autophagy in diabetic nephropathy. J Endocrinol 224.
- Huber TB, Walz G, Kuehn EW (2011) mTOR and rapamycin in the kidney: signaling and therapeutic implications beyond immunosuppression.Kidney International 79: 502–511.
- Thomas MC (2014) Renal effects of dapagliflozin in patients with type 2 diabetes. Therapeutic Advances in Endocrinology and Metabolism 5: 53-61.
- Wanner C,Inzucchi SE, John M, Lachin JM,Fitchett D, et al. (2016) For the EMPA-REG OUTCOME Investigators.Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. New Engl J of Med 1-12.
- Kuecker CM, Vivian EM (2016) Patient considerations in type 2 diabetes – role of combination dapagliflozin–metformin XR.Diabetes MetabSyndrObes 9: 25–35.
- Morita T, Nakano D, Kitada K, Morimoto S, Ichihara A, et al. (2015) Chelation of dietary iron prevents iron accumulation and macrophage infiltration in the type I diabetic kidney. Eur J Pharmacol 756: 85–91.
- Zou C, Liu X, Liu R, Wang M, Sui M, et al. (2016) Effect of the oral iron chelatordeferiprone in diabetic nephropathy rats. Diabetes.
- Tharaux PL, Huber TB (2016) How Is Proteinuric Diabetic Nephropathy Caused by Disturbed Proteostasis and Autophagy in Podocytes? Diabetes 65: 539-541.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences