Translational Nephrology: Taking Aim at Tubular Debris
Jonathan A. Lindquist and Peter R. Mertens
DOI10.21767/2472-5056.100012
Jonathan A. Lindquist* and Peter R. Mertens
Department of Nephrology, Hypertension, Diabetes and Endocrinology, Otto-von-Guericke University, Leipziger Strasse 44, 39120 Magdeburg, Germany
- *Corresponding Author:
- Jonathan A. Lindquist
Hypertension, Diabetes and Endocrinology
Otto-von-Guericke University
Leipziger Strasse 44
39120 Magdeburg, Germany
Tel: +49-391-6724703
E-mail: Jon.Lindquist@med.ovgu.de
Received date: April 04, 2016; Accepted date: May 18, 2016; Published date: May 20, 2016
Citation: Lindquist JA, Mertens PR (2016) Translational Nephrology: Taking Aim at Tubular Debris. J Clin Exp Nephrol 1:12. DOI:10.21767/2472-5056.100012
Copyright: © 2016 Lindquist JA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License,which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Acute kidney injury is characterized by a rapid decline in renal function, which results in the accumulation of metabolic waste and toxins. Changes that result in decreased renal blood flow or the presence of toxic chemicals (e.g. metabolic by-products from medications) within the blood can cause stress to the tubular cells within the kidney, whose task is to reabsorb metabolites before they are excreted in the urine. If the stress persists, tubular cells will die. The loss of cells within the tubules decreases the kidneys capacity for reabsorption, and the debris produced by dying cells can result in blockage of the tubules, resulting in decreased flow. The combination of these factors within the nephrons, decreased flow, increased renal protein, high salt, and low pH create ideal conditions for cast formation, which can further complicate matters. Therefore, mechanisms are needed to ensure efficient clearance of debris in order to initiate repair and restore kidney function. In their recent paper, Arai et al. (Nat. Med. 2016; 22: 183-193), have identify one such mechanism involving the Apoptosis Inhibitor of Macrophages protein (AIM). In the blood of healthy individuals, AIM can be found in complexes with IgM. The presence of inflammation induces the release of AIM, which then enters into the tubules within the kidney to mark debris for disposal by phagocytes. Activated tubular cells, which are undergoing proliferation in order to replace lost cells, upregulate a receptor that binds AIMtagged debris. The receptor is called Kidney Injury Molecule 1 (KIM-1) and bestows upon the surviving tubular cells the ability to repair tubular function much like a do-it-yourself (DIY) kit. Best of all, it appears that the application of recombinant AIM during AKI may be of therapeutic benefit for improving kidney function.
Keywords
Acute kidney injury; Apoptosis inhibitor of macrophage protein; Ischemia/ reperfusion injury; Kidney injury molecule-1; Proximal tubular epithelial cells
Abbreviations
AIM: Apoptosis Inhibitor of Macrophage protein; AKI: Acute Kidney Injury; IgM: Immunoglobulin μ Chain; IgV: Immunoglobulin Variable domain; IRI: Ischemic Reperfusion Injury; KIM-1: Kidney Injury Molecule-1; LPS: Lipopolysaccharide; PTEC: Proximal Tubular Epithelial Cells
Mechanisms of acute Kidney Injury
Acute kidney injury (AKI) represents a global health problem associated with high morbidity and mortality. AKI has multiple causes, including reduced renal blood flow (ischemia), nephrotoxicity, as well as urinary tract obstruction. Despite its diverse causes, the common element is tubular cell damage, which induces inflammation. If left untreated, fibrosis develops that progresses to chronic kidney disease (CKD) and ultimately ends in renal failure. Unfortunately, aside from renal replacement therapy and kidney transplantation, no reliable treatment exists that improves survival and restores kidney function [1]. While animal models, such as ischemiareperfusion injury and nephrotoxicity, have provided detailed insight into the mechanisms of damage, they have so far done little to improve therapy; until now.
The kidney’s primary function is to remove metabolic waste products from the blood; however it also plays an important role in homeostasis, regulating the levels of electrolytes, maintaining the acid-base balance, as well as regulating blood pressure. Filtration of the blood occurs within the glomeruli. Nitrogenous wastes are secreted in the urine, while water, salts, carbohydrates, and amino acids are reabsorbed within the tubules before they reach the collecting ducts; this accounts for more than two-thirds the volume of the filtrate. Resorption is an active process that requires ATP. Therefore tubular cells need ample numbers of mitochondria in order to meet their energy demands. This energy dependency makes them extremely susceptible to decreases in blood flow (ischemia), which result is reduced oxygen levels (i.e. hypoxia). Additionally, due to the uptake of proteins and salts, tubular cells are also extremely sensitive to cellular stress. Stress occurs when protein levels rise (e.g. during proteinuria) leading to an unfolded protein response (UPR) or when toxic levels of drugs are achieved within the cell. If the stress is not removed, injured cells will die, either by apoptosis (programmed cell death) or necrosis. Necrotic cell death releases cytoplasmic and nuclear components (e.g. ATP or the chromatin-associated protein high-mobility group box 1 (HMGB1)), which are recognized as danger signals [2,3]. Dead cells slough off and are washed away by the flow; however this may result in blockage particularly where the distal loops of the tubules narrow.
The release of danger signals results in inflammation, recruiting immune cells from the blood into the kidney as well as in the activation of the surviving tubular cells. Activated tubular cells undergo changes in morphology, e.g. loss of brush border membranes, flattening of the epithelium, and they begin to migrate and proliferate in order to reestablish membrane integrity and thereby restore organ function [4]. Experimental models of ischemia/reperfusion injury (IRI) have clearly demonstrated the regenerative potential of the kidney [5]. The challenge, however, is learning how to harness this.
What Role do Tubular Casts Play in Acute Kidney Injury?
In addition to the morphological changes observed for injured tubular cells, cast formation within the tubules, as well as dilation of the lumen is observed. Casts are cylindrical structures that are either cellular or acellular in origin. Cellular casts may be composed of erythrocytes, leukocytes, bacteria, or epithelial cells. The formation of these casts is often associated with specific conditions (e.g. pylonephritis or nephrotoxicity). Acellular casts are formed from urinary proteins or solutes.
The loss of tubular cell resorption results in accumulation of protein(s) and salts within the urinary filtrate. This combined with the reduced flow caused by debris blockage creates ideal conditions for cast formation. Uromodulin, also known as Tamm-Horsfall glycoprotein (THP), is produced by tubular cells within the loop of Henle. In addition to being the most abundant excreted protein in the urine, it is also highly susceptible to precipitation under these conditions (low flow, high salt, low pH) to form hyaline casts; the most common form of cast observed. Serum proteins, such as albumin or immunoglobulin light chains, are also susceptible to precipitation under these conditions and lead to the formation of granular casts; the second-most common form.
Additional types of acellular casts include waxy casts, fatty casts, pigmented, and/or crystal casts; the latter being formed of crystallized solutes within the urine. These casts are thought to be indicators of chronic kidney disease and represent cast evolution, due to their larger size and the inclusion of fat globules, cholesterol, or metabolic breakdown products such as hemoglobin, myoglobin, or bilirubin.
Tubular Cell Death: Necrosis Versus Apoptosis?
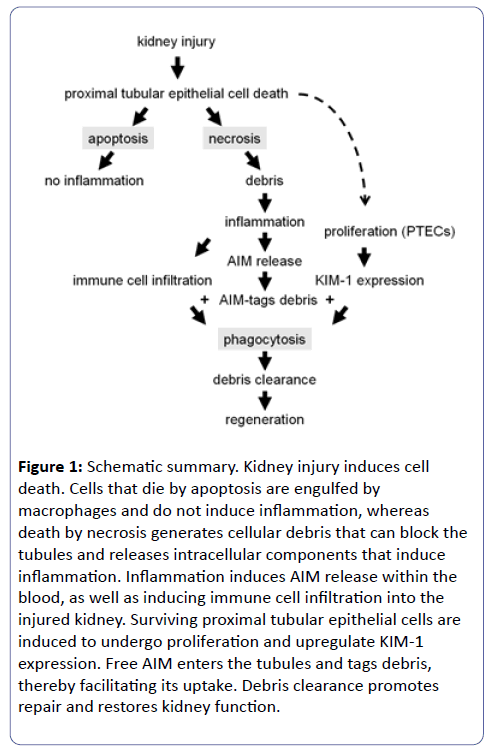
If the stress is not removed, injured tubular cells will die. The path ‘chosen’, either apoptosis or necrosis, depends largely on the type of stress (Figure 1). Animal models of albuminuria have shown that excessive protein resorption induces an unfolded protein response within tubular cells, which activates cell intrinsic pathways leading to apoptosis [6]. Alternatively, models of cisplatin nephrotoxicity have shown that the induction of necrosis versus apoptosis is dependent upon the dosage. Recently, a number of regulated necrosis pathways, including necroptosis, pyroptosis, and ferroptosis have been identified [7].
Figure 1: Schematic summary. Kidney injury induces cell death. Cells that die by apoptosis are engulfed by macrophages and do not induce inflammation, whereas death by necrosis generates cellular debris that can block the tubules and releases intracellular components that induce inflammation. Inflammation induces AIM release within the blood, as well as inducing immune cell infiltration into the injured kidney. Surviving proximal tubular epithelial cells are induced to undergo proliferation and upregulate KIM-1 expression. Free AIM enters the tubules and tags debris, thereby facilitating its uptake. Debris clearance promotes repair and restores kidney function.
To Form Debris that is Removed by Phagocytosis or Washed Away?
The mechanism of cell death also impacts upon debris formation. Apoptosis is a tightly regulated pathway. Dying cells produces ‘eat me’ signals that recruit phagocytes to ensure an efficient and tidy removal. Whereas regulated necrosis pathways can result in the death of single cells or, in some cases, the synchronized destruction of an entire tubular segment [8]. The rapid demise of a large number of cells within one tubule can generate sufficient debris to result in tubular obstruction. Thus, mechanisms are required in order to ensure efficient debris removal in order to restore tubular function. One aspect of this model that is not clear is whether the restricted urinary flow caused by debris blockage contributes in any way to cellular proliferation and tissue regeneration.
Summary of Key Findings
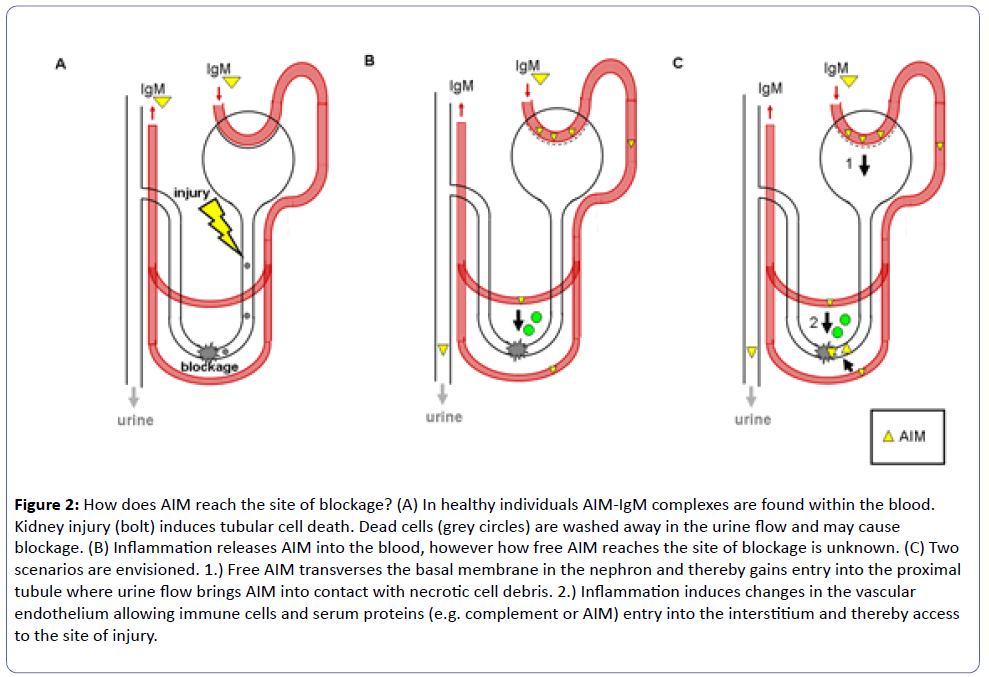
The good news for today: A remedy for acute kidney injury is in sight. In a recent paper, Arai et al. demonstrate the therapeutic benefits of using recombinant Apoptosis Inhibitor of Macrophage protein (rAIM) to treat kidney injury. Using a bilateral model of ischemia/reperfusion injury (IRI), the authors observed that mice deficient for the gene CD5 molecule-like (Cd5l), also known as AIM, show more severe injury and enhanced mortality in comparison to wild-type mice. Acute kidney injury (AKI) results in the death of proximal tubular epithelial cells (PTECs), this debris is then washed away in the urine, but can cause blockage in the collecting ducts on its way to the ureter (see Figure 2). Surviving tubular epithelial cells must then proliferate to initiate repair and restore kidney function. AIM-deficiency did not influence tubular cell proliferation or the rate of cell death, leading the authors to hypothesize that AIM must be involved in debris clearance. Indeed, immunohistochemistry demonstrated AIM deposited within the debris at the cortico-medullary junction. Due to similarities in the phenotype with that of mice missing the hepatitis A virus cellular receptor 1 (Havcr1) gene, also known as kidney injury molecule-1 (KIM-1), the authors hypothesized that these proteins might overlap in function. Indeed further investigations established AIM as a ligand of KIM1, which facilitated the uptake of necrotic cell debris by KIM-1 expressing cells, i.e. activated/proliferating tubular epithelial cells. Additionally, coating of necrotic cell debris with rAIM also facilitated its uptake by macrophages. Interestingly, although AIM is produced by macrophages, it appears that the reservoir of extracellular AIM associated with circulating IgM is essential for repair and survival. Finally, the application of rAIM into injured AIM-deficient mice facilitated debris clearance, thereby reducing injury markers and enhancing survival. Thus, rAIM may be an appropriate means to target tubular debris.
Figure 2: How does AIM reach the site of blockage? (A) In healthy individuals AIM-IgM complexes are found within the blood. Kidney injury (bolt) induces tubular cell death. Dead cells (grey circles) are washed away in the urine flow and may cause blockage. (B) Inflammation releases AIM into the blood, however how free AIM reaches the site of blockage is unknown. (C) Two scenarios are envisioned. 1.) Free AIM transverses the basal membrane in the nephron and thereby gains entry into the proximal tubule where urine flow brings AIM into contact with necrotic cell debris. 2.) Inflammation induces changes in the vascular endothelium allowing immune cells and serum proteins (e.g. complement or AIM) entry into the interstitium and thereby access to the site of injury.
Apoptosis Inhibitor of Macrophage (AIM) Protein
AIM was identified for its ability to inhibit apoptosis [9]. AIM is secreted by macrophages, with notable expression seen in the liver, spleen, lymph nodes, and bone marrow. AIM secretion is enhanced by inflammatory stimuli, e.g. LPS. However, AIM is not found as a free protein in the serum of healthy individuals, but rather in a complex with IgM pentamers. Indeed efforts are being made to stabilize circulating AIM with synthetic IgM-Fc [10]. To further emphasize the importance of the AIM-IgM complex, Aria et al. performed IRI in mice lacking secreted IgM (Δsμ). Here, AIM was no longer detectable in the debris deposits, even though macrophages still express the protein. The Δsμ mice showed an enhanced mortality similar to that seen in both AIM- or KIM-1-deficient animals.
Kidney Injury Molecule-1 (KIM-1)
KIM-1, the product of the hepatitis A virus cellular receptor 1 (Havcr1) gene, is a type I transmembrane glycoprotein. It possesses an IgV and a mucin domain, both of which are needed for its interaction with AIM [11]. However whether AIM binds to the junction of these two domains, at a site whose structure is perhaps reminiscent of IgM (i.e. Ig domain plus glycosylation) has not been demonstrated, nor has a direct interaction between these proteins.
KIM-1 expression is not detected in healthy kidney tissue, however its expression is markedly upregulated in PTECs in response to injury via a STAT3-dependent mechanism [12,13]. KIM-1 recognizes both phosphatidylserine and oxidized lipoproteins. KIM-1 expression enables activated tubule cells to engulf debris from dead cells, thus conferring the status of semi-professional phagocyte. This phagocytic activity is important for debris clearance, as KIM-1-deficient mice demonstrate elevated levels of injury markers, cellular debris, and increased mortality [11,14,15].
Using a phagocytosis assay, Arai et al. show that AIM does not influence the uptake of apoptotic cells by KIM-1 expressing proximal tubular cells; however AIM coating does facilitate the phagocytosis of necrotic cell debris. AIM-coating also improved the uptake of necrotic cell debris by macrophages, which do not express KIM-1. Therefore other scavenger receptors, e.g. CD36, must also be involved [16]. However, given that CD36-deficient mice showed minimal differences in the IRI model, the authors considered the contribution of CD36 to be marginal for this injury model.
KIM-1 is also shed from the surface of activated PTECs by metalloproteinases, such as the TNF-α-converting enzyme (TACE or ADAM17) [17-19]. This activity is responsible for releasing KIM-1 into the urine, which led to its suggested usefulness as a biomarker [12]. KIM1 expression has also been detected in individuals with chronic renal failure and transgenic expression of KIM-1 has been shown to induce fibrosis [20-22]. Thus it appears that a tightly regulated temporal expression of KIM-1 within the kidney is important for proper function.
Discussion and Outlook
Arai et al. demonstrate that AIM is released from its IgM reservoir upon injury, however how free AIM gets from the blood to the site of injury has not been established (see Figure 2). The obvious path would be to go with the flow, i.e. directly via the nephron filter. Given that the predicted molecular weight of AIM is smaller than that of albumin (37 kDa versus 66 kDa, respectively) and that injury often negates the charge barrier of the basal membrane enhancing its permeability to serum proteins, this route is certainly feasible.
However, infiltrating immune cells choose a different path, exploiting inflammation-induced changes within the endothelium (e.g. the upregulation of adhesion proteins) to transmigrate via the interstitium to reach the site of injury [23]. Given that other serum proteins, e.g. complement, are also observed in this compartment, this scenario must also be considered. Thus, it appears that further investigations are required to resolve this issue.
Regardless of the pathway used, AIM is present both at the sites of blockage as well as in the urine. Since AIM tags debris, it may be of interest to determine whether it is also present in urinary casts and whether this has additional diagnostic potential. Since the ectodomain of KIM-1 is also shed into urine [17-19], a more careful analysis of the kinetics of AIM and KIM-1 secretion are needed. One can easily envision that shedding of KIM-1 soon after injury could be deleterious to the beneficial effects of AIM and therefore could be a contributing factor in cast formation. AIM is not detected in the urine of healthy individuals or mice; however protein levels peaked 1 day after IRI and then return to baseline. Although AIM is new to the field as a kidney injury marker, serum AIM has been identified as a potential marker of other inflammatory conditions [24-26]. KIM-1 has also received much attention as a biomarker for kidney injury, however despite its identification more than 15 years ago; it has as of yet failed to make the transition into the clinic [12,19,27].
Take Home Message
Early detection of kidney injury should facilitate treatment. Advancements in this respect are certainly welcome. However, the central problem remains the lack of effective therapy. The hallmark of this paper is the demonstration that application of recombinant AIM not only tags debris, but also facilitates its clearance thereby restoring kidney function and improving survival (see Figure 1). Validation of AIM’s efficacy in other injury models is eagerly awaited and many eyes will certainly be looking to see if AIM can make the jump from bench to bedside.
Supplementary Discussion (for Researchers)
KIM-1 is also reportedly expressed on plasmacytoid dendritic cells and germinal center B-cells within the spleen (www.immgen.org). Whether the AIM-KIM-1 interaction plays a role in homeostasis by clearing apoptotic debris from the blood or perhaps in immune surveillance by processing AIMcoated necrotic debris during infection or other inflammatory conditions has yet to be explored.
Activated T cells also express KIM-1 and infiltrate into the injured kidney. Whether they see AIM-tagged debris and if so, what role they play has yet to be determined. From macrophages, we know that phagocytosis of apoptotic debris favors an anti-inflammatory phenotype. Does this ‘rule’ apply to activated PTECs? And if so, do they also secrete antiinflammatory cytokines to limit inflammation and promote healing? These questions certainly merit further investigation.
Acknowledgements
This work was funded by DFG grants LI-1031/4-1 to JAL, and ME1365/7-2, ME-1365/9-1, and SFB854 TP-A01 to PRM. The authors would like to thank Dr. Josephine Hildebrandt for helpful discussion.
References
- Zuk A, Bonventre JV (2016) Acute Kidney Injury. Annu Rev Med 67:293-307.
- Gallucci S, Matzinger P (2001) Danger signals: SOS to the immune system. CurrOpinImmunol13:114-119.
- Bianchi ME, Manfredi AA (2007) High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev220:35-46.
- Devarajan P (2006) Update on mechanisms of ischemic acute kidney injury. J Am SocNephrol17:1503-1520.
- Ghielli M, Verstrepen W, Nouwen E, De Broe ME (1998) Regeneration processes in the kidney after acute injury: role of infiltrating cells. ExpNephrol 6:502-507.
- Ding LH, Liu D, Xu M, Wu M, Liu H, et al. (2015) TLR2-MyD88-NF-kappaB pathway is involved in tubulointerstitial inflammation caused by proteinuria. The international journal of biochemistry & cell biology69:114-20.
- Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, et al. (2014) Regulated cell death in AKI. J Am SocNephrol25:2689-2701.
- Linkermann A (2016)Nonapoptotic cell death in acute kidney injury and transplantation. Kidney international89:46-57.
- Miyazaki T, Hirokami Y, Matsuhashi N, Takatsuka H, Naito M (1999) Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J Exp Med189:413-422.
- Kai T, Yamazaki T, Arai S, Miyazaki T (2014) Stabilization and augmentation of circulating AIM in mice by synthesized IgM-Fc. PLoS One9:e97037.
- Arai S, Kitada K, Yamazaki T, Takai R, Zhang X, et al. (2016) Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med 22:183-193.
- Bonventre JV (2009) Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant24:3265-3268.
- Ajay AK, Kim TM, Ramirez-Gonzalez V, Park PJ, Frank DA, et al. (2014) A bioinformatics approach identifies signal transducer and activator of transcription-3 and checkpoint kinase 1 as upstream regulators of kidney injury molecule-1 after kidney injury. J Am SocNephrol25:105-118.
- Yang L, Brooks CR, Xiao S, Sabbisetti V, Yeung MY, et al. (2015) KIM-1-mediated phagocytosis reduces acute injury to the kidney. J Clin Invest125:1620-1636.
- Xiao S, Brooks CR, Zhu C, Wu C, Sweere JM, et al. (2012) Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Igmucin 1 (Tim-1) mucin domain-mutant mice. ProcNatlAcadSci U S A109:12105-12110.
- Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13(9):621-34. doi: 10.1038/nri3515. PubMed PMID: 23928573.
- Gandhi R, Yi J, Ha J, Shi H, Ismail O, et al. (2014) Accelerated receptor shedding inhibits kidney injury molecule-1 (KIM-1)-mediated efferocytosis. Am J Physiol Renal Physiol307:F205-221.
- Zhang Z, Humphreys BD, Bonventre JV (2007) Shedding of the urinary biomarker kidney injury molecule-1 (KIM-1) is regulated by MAP kinases and juxtamembrane region. J Am SocNephrol18:2704-2714.
- Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, et al. (2002) Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J BiolChem277:39739-39748.
- Vaidya VS, Waikar SS, Ferguson MA, Collings FB, Sunderland K, et al. (2008) Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. ClinTranslSci1:200-208.
- van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, et al. (2007) Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol212:209-217.
- umphreys BD, Xu F, Sabbisetti V, Grgic I, MovahediNaini S, et al. (2013) Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest123:4023-4035.
- Jang HR, Rabb H (2015) Immune cells in experimental acute kidney injury. Nat Rev Nephrol11:88-101.
- Yamazaki T, Mori M, Arai S, Tateishi R, Abe M, et al. (2014) Circulating AIM as an indicator of liver damage and hepatocellular carcinoma in humans. PLoS One9:e109123.
- Mera K, Uto H, Mawatari S, Ido A, Yoshimine Y, et al. (2014) Serum levels of apoptosis inhibitor of macrophage are associated with hepatic fibrosis in patients with chronic hepatitis C. BMC Gastroenterol14:27.
- Miyazaki T, Kurokawa J, Arai S (2011)AIMing at metabolic syndrome. -Towards the development of novel therapies for metabolic diseases via apoptosis inhibitor of macrophage (AIM). Circ J75:2522-2531.
- Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, et al. (1998) Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J BiolChem273:4135-4142.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences