The Impact of Inflammation on Cognitive Impairment in Chronic Kidney Disease Patients
Antonia Kaltsatou
DOI10.21767/2472-5056.100020
Antonia Kaltsatou*
School of Physical Education & Sport Science, University of Thessaly, Trikala, Greece
- *Corresponding Author:
- Antonia Kaltsatou
School of Physical Education & Sport Science, University of Thessaly
Trikala, Greece
Tel: +43-1-47654-6294
E-mail: kaltsatou@yahoo.com
Received date: May 30, 2016; Accepted date: October 25, 2016; Published date: October 28, 2016
Citation: Kaltsatou A (2016) The Impact of Inflammation on Cognitive Impairment in Chronic Kidney Disease patients. J Clin Exp Nephrol 1:20. doi:10.21767/2472-5056.100020
Copyright: © 2016 Kaltsatou A. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Cognitive impairment is a common characteristic in Chronic Kidney Disease (CKD) patients. It has been suggested that inflammation may be implicated in cognitive impairment found in CKD patients. Hence, the aim of this systematic review was to provide an update on recent advances in our understanding of how inflammation contributes to cognitive impairment in uremic patients. Methods: A systematic review was conducted searching Pubmed and Scopus by using the Cochrane and PRISMA guidelines from March 2016 through August 2016. PubMed, Science Direct, Scopus, and Google Scholar were used to search for studies that investigated the impact of inflammation in cognitive impairment in CKD patients. Results: The literature search identified only two studies that examined inflammatory biomarkers and cognitive impairment, and correlated them, indicating that inflammation is implicated in cognitive impairment in CKD patients. It has been suggested that cognitive impairment is related to the level of renal functional impairment and especially in the end stage renal disease, where the immune system is strongly activated, cognitive impairment is more profound. Cytokines, a major contributor to molecular mechanisms, maintain memory and cognitive processes and may impair hippocampal plasticity. Cytokine production may have a different negative effect and may affect the early stages of cell production and proliferation in the dentate gyrus. Conclusion: Inflammation may be implicated in cognitive impairment found in CKD patients. However, further research should be done in this field in order to investigate the mechanisms that are implicated in cognitive impairment in CKD patients.
Keywords
Inflammation; Cognitive impairment; Chronic kidney disease; Hemodialysis
Introduction
Chronic Kidney Disease (CKD) is a major public health problem with a continuously increasing prevalence [1]. It has been estimated that between 1990 and 2010 the mortality rate of CKD has increased significantly and CKD, which occupied the 27th position of causes of death, has now reached the 18th position [2]. As glomerular filtration rate (GFR) decreases, patients are progressively lead to end-stage renal disease (ESRD), where clinical abnormalities are more profound. At ESRD, the kidney function is significantly reduced to a level less than 10% and a renal replacement therapy of hemodialysis (HD) is necessary. Although during the last decades the technology and the effectiveness of HD therapy have improved, HD therapy still induces numerous implications, which are responsible, to some extent, for the significant clinical problems that CKD patients experience. Furthermore, according to the ERA-EDTA annual report of 2013, it was estimated that the 5-year adjusted survival of all patients who received the HD therapy was 60.9% [3].
Patients with CKD, and especially those in ESRD, apart from the hazardous metabolic and physiological abnormalities that they experience, they also suffer from mental health deficits. Specifically, it has been demonstrated that compared to healthy subjects the risk for cognitive impairment development is higher in CKD patients at all stages [4]. However, cognitive impairment is more profound in ESRD patients who receive the treatment of HD [5]. More precisely, it has been estimated that only a minor percentage of HD patients’ present normal cognitive function [6,7] and a percentage between 30%-60% who receive the HD therapy, suffer from cognitive impairment [4,8].
Cerebral ischemia caused by cardiovascular and hemodynamic abnormalities has been proposed to be a major contributor to impaired cognitive activity in HD patients [9,10]. Moreover, anaemia has been related to poor cognitive performance [11] and the fact that cognitive function has been improved after the correction of anaemia in HD patients, enhances this hypothesis [12]. Secondary hyperparathyroidism [13,14], dialysis disequilibrium [15] and uremic toxins [16] are also believed to contribute to cognitive impairment that characterizes CKD patients. Specifically, in a systematic review article by Watanabe et al. [16], where the relationship between uremic toxins and cerebro-renal interaction dysfunction was reviewed, the authors searched the literature for 21 uremic toxins. They concluded that among these compounds uric acid, indoxyl sulfate, p-cresyl sulphate, interleukin 1-β, interleukin 6, TNF-α and parathyroid hormone affected the cerebro-renal interaction dysfunction [16]. Especially, elevated levels of indoxyl sulfate have been related to reduced executive function in early stages of CKD, and possibly the early removal of indoxyl sulfate may contribute to cognitive impairment prevention [17].
Inflammation is also believed to affect cognitive function. Specifically, inflammation has been suggested to be an aetiological factor of mild cognitive impairment [18]. More precisely, a relation between the levels of C-reactive protein (CRP) and interleukin-6 (IL-6) with reduced cognitive performance in healthy subjects and in older adults, has been suggested [19]. In addition, in HD patients it has also been found that abnormal production of cytokines is associated with cognitive impairment [20]. Severe or prolonged systemic inflammation can induce harmful changes in cognitive function such as synaptic loss, dendritic alterations, neuronal apoptosis, suppression of BDNF, impaired neurogenesis, memory dysfunction, and altered hypothalamic function by activating microglia [21]. Thus, the aim of this systematic review is to provide an update on recent advances in our understanding of how inflammation contributes to cognitive impairment in CKD patients.
Methods
A systematic review was conducted searching Pubmed and Scopus by using the Cochrane and PRISMA guidelines from March 2016 through August 2016. PubMed, Science Direct, Scopus, and Google Scholar were used to search for studies that investigated the impact of inflammation in cognitive impairment in CKD patients. Eligibility of the studies based on titles, abstracts and full-text articles was determined. Studies were selected using inclusion and exclusion criteria. In the current review, only studies that met the following criteria were included: studied CKD patients and patients on HD treatment, assessed cognitive function in CKD patients; addressed randomized control trials or controlled trials or clinical trials or pilot studies designed to evaluate the impact of inflammation in cognitive impairment in CKD patients, lack of drug or diet interventions in the brain or lack of uremic animal models. Studies were excluded when they referred to patients on peritoneal dialysis and concerned low quality studies, namely studies with methodological flaws or lack of reporting.
Results
Although cognitive impairment is a common abnormality for CKD patients, and it has been suggested that inflammation is implicated in cognitive dysfunction, the literature search revealed only two studies that examined the impact of inflammation in cognitive impairment in CKD patients. More precisely, the literature search identified a total of 28 articles. Of these articles, only two studies met the inclusion criteria that were set in this review. Articles were excluded for the following reasons: they did not examine the impact of inflammation in cognitive impairment in CKD patients, or they were review articles. Consequently, from the two studies that were identified by the literature search, only one study examined cytokine production and correlated it with psychological alterations and quality of life in CKD patients, and another study where CRP levels and cognitive impairment was associated in CKD patients during the hemodialysis session.
Discussion
Inflammation and cognitive impairment
Inflammation is the acute response of the body that protects the organism against invading microorganisms, limits tissue damage, and maintains homeostasis. The fact that the incidence for Alzheimer’s disease was limited in patients with rheumatoid arthritis, who received anti-inflammatory medication, generated the conclusion that inflammation is an etiological factor for cognitive impairment [22,23]. Specifically, McGeer et al. [23] conducted a systematic review where seventeen epidemiological studies were reviewed. In this review the hypotheses that brain inflammation is considered as the main cause for neuronal injury in Alzheimer’s disease and that antiinflammatory medication could have protective properties, were examined [23]. The authors concluded that anti-inflammatory drugs had a protective effect against Alzheimer’s disease and accordingly that inflammation is a major factor for cognitive decline [23]. Generally, some cytokines have been related with cognitive impairment and dementia [24] and more precisely interleukin 1beta (IL-1-β), interleukin 6 (IL-6) and tumor necrosis factor (TNF) have been associated with Alzheimer’s disease [24,25].
In the next decades many studies examined and proved the relation between inflammation and cognitive impairment. Specifically, it has been found that increased levels of IL-6 and CRP were negatively associated with global cognition and executive function in aged subjects [26,27]. Moreover, elevated levels of IL-6 were related to reduced cognitive performance in a multiethnic stroke free cohort of 3,298 people [28]. Specifically, the Northern Manhattan study examined the relation between plasma IL-6 levels and cognitive deficit, where vascular risk factors, apolipoprotein ε4 (APOE4) allele and inflammation were taken into consideration [28]. The results of this study revealed that cognitive deficit was more profound in the participants with IL-6 values above the median compared to those with IL-6 levels below the median [28]. Moreover, a relation among age, cognitive deficit and IL-6 levels was also observed as the effect of IL-6 on cognitive dysfunction was significant only in the participants with age >71 years [28]. Furthermore, it was found that vascular risk factors did not influence the relation between IL-6 and cognitive decline, while after adjusting for APOE4 allele this relation was not significant, indicating a strong intercession [28].
In addition, in a study by Yaffe et al. [29], where the relationship between metabolic syndrome and cognitive decline was examined, it was found that metabolic syndrome is a risk factor for cognitive impairment, due to elevated inflammation. Indeed, the results of this study revealed that older subjects with metabolic syndrome, who were characterized by increased levels of IL-6 and CRP, showed significantly reduced scores on the modified Mini Mental State Examination, indicating a relation between inflammation and global cognitive decline [29]. However, in the Northern Manhattan study it was found that high sensitivity CRP did not influence the cognitive decline. Furthermore, in a study by Trollor et al. [30], where the inflammatory markers of CRP, interleukin -1β, -6, -8, -10 and -12 were examined, a relation between mild cognitive impairment and inflammatory markers was revealed. According to all these studies, more frequently CRP and IL-6 have been found to correlate with impaired cognitive function.
IL-6 is related with the risk of atherosclerosis [31,32] and cardiovascular disease [33,34] development. IL-6 and its receptors (IL-6R) are located in neurons in different brain regions such as hippocampus, hypothalamus, neocortex and cerebellum [35] and released by astrocytes [36], microglia [37] and neurons [38]. In healthy subjects IL-6 is implicated in many physiological functions within the Central Nervous System (CNS). It has been found that IL-6 regulates neurogenesis, and IL-6 over-express contributed to a severe reduction in neurogenesis in the hippocampal dentate gyrus in adult transgenic mice [39]. These results are in accordance with those of other studies, which observed impaired learning and memory due to increased IL-6 levels [40]. The results of these studies indicate that elevated levels of IL-6, as happens during aging, probably contribute to cognitive impairment by reducing neurogenesis. Generally, neurogenesis is the physiological function that regulates memory function and contributes to memory consolidation [41] and to hippocampal-dependent learning ability [42]. However, Hryniewicz et al. [43], investigated whether there is an involvement of endogenous IL-6 in recognition memory in mice, who lack a functional gene for this cytokine. These authors [43] found that IL-6 deficiency induces a distracting effect in the recognition memory of this kind of mice. A systematic review by Tso et al. [44], where studies that examined the relation between IL-6 and the IL-6 -174G/C polymorphism, supported that IL-6 elevated levels are associated with a higher risk of stroke. However, there are conflicting results in the literature and it remains unclear whether an association between the IL-6 -174G/C polymorphism and the ischemic cerebrovascular events exists, due to the complexity of the IL-6 physiology [44]. The results of all these studies prove that IL-6 plays an important role in cognitive function.
Except neurogenesis, IL-6 has been proved to influence synaptic plasticity [45]. Generally, cytokines are a major contributor to molecular mechanisms, which maintain memory and cognitive processes [46,47], and may impair hippocampal plasticity. It has been suggested that cytokine production has a different negative effect and may affect the early stages of cell production and proliferation in the dentate gyrus [48].
Specifically, pro-inflammatory mediators disturb hippocampal neuronal functions, and accordingly affect long-term potentiation and working memory consolidation [49,50]. Moreover, in an animal study by Seguin et al. [48], where the impact of systemic and intra-hippocampal infusion of the IL-1β, IL-6 and TNF-α over the generation of new dentate gyrus cells in male mice was examined, the authors concluded that systemic TNF-α decreased 5-bromo-2-deoxyuridine (BrdU) in the hippocampus. These results indicate that the inflammatory marker of TNF-α, had a negative effect in cellular proliferation by acting upon peripheral targets suggesting that acute systemic cytokine exposure alters hippocampal cell proliferation and neurogenesis [48]. Furthermore, a single and repeated central cytokine infusion differentially influences hippocampal cell proliferation [48].
The Janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway are commonly activated by cytokines. Both JAKs and STATs regulate neuronal proliferation, survival and differentiation [51]. When the cytokine release and the binding of a cytokine to its cell-surface receptor occurs, this function has as a consequence in the dimerization of the receptor and it accordingly activates JAK tyrosine kinases, which are related to the receptor. Then the activated JAKs phosphorylates the tyrosine residues, which are on the receptor and serve as docking sites for STATs. Accordingly, JAKs phosphorylate STATs, which are then being dimerized, leave the receptor and translocate to the nucleus and activate gene transcription [52,53].
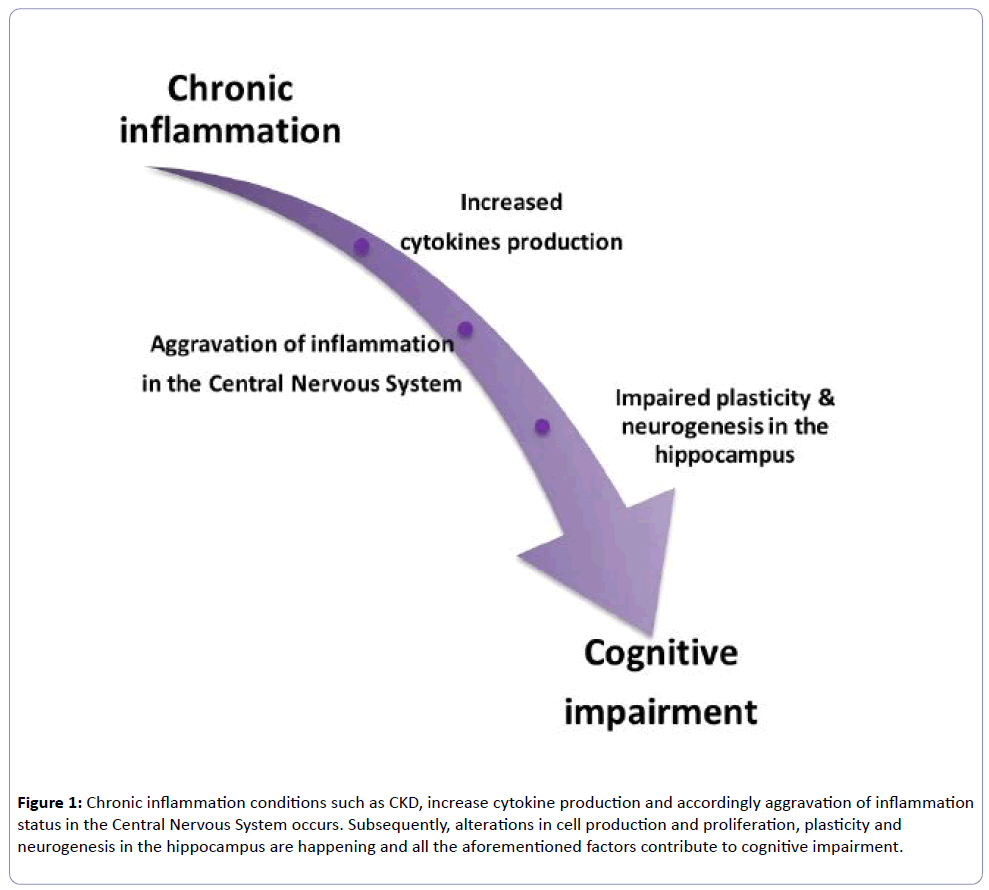
JAKs activate the STATs and JAK1 is the major activator of STAT3, which activates nuclear STAT3, and has been detected in many abnormalities. Moreover, from the cytokines family, IL-6 and IL-10 activate STAT3, which is a member of the STAT family and contributes to cell growth, differentiation and survival signals [54]. It has been found that after the completion of 4 hours of cells exposure in IL-6, STAT3 is rephosphorylated and remains active for many hours [55]. The main consequence of the prolonged activation of STAT3, is that this abnormality leads to chronic inflammatory diseases [55]. There is evidence that STAT3 regulates the physiological status of mature neurons and mediates cell growth, differentiation and survival signals [56]. In light of all these data, inflammation is implicated in cognitive impairment and can be considered a major contributor to cognitive decline development (Figure 1).
Figure 1: Chronic inflammation conditions such as CKD, increase cytokine production and accordingly aggravation of inflammation status in the Central Nervous System occurs. Subsequently, alterations in cell production and proliferation, plasticity and neurogenesis in the hippocampus are happening and all the aforementioned factors contribute to cognitive impairment.
Impact of inflammation in cognitive impairment in CKD patients
The literature search identified only one study by Montinaro et al. [20] that examined inflammatory biomarkers and cognitive impairment and correlated them. Montinaro et al. [20], who investigated the psychological alterations in HD patients and correlated them with cytokine production, observed that an association between abnormal cytokine production and the presence of emotional symptoms existed. In this study the intervention group consisted of 30 HD patients with a minimum dialysis age of three months while 20 patients with CKD stage I-II were used as controls. Cognitive impairment was assessed with the modified version of the Kidney Disease Quality of Life (KDQOL) questionnaire, which included a cognitive function subscale. At the beginning of the HD therapy, blood samples were collected and the inflammatory biomarkers of IL-1β, IL-6, TNF-α and IL-10 were examined. The results of this study revealed that the HD patients had increased levels of all the inflammatory biomarkers compared to controls. Specifically, HD patients showed increased levels of IL-1β by 48.5%, IL-6 by 51.4%, TNF-α by 100.1% and IL-10 by 153.85% compared to controls. Moreover, an inverse correlation between the score at the cognitive function subscale of the KDQOL questionnaires and IL-6, TNF-α and IL-10 was revealed, indicating that inflammation is a major contributor to cognitive impairment that characterizes ESRD patients [20]. These findings are in line with a study by van den Kommer [57], suggesting that inflammation in combination with other risk factors are implicated in cognitive dysfunction.
Patients with CKD are characterized by elevated levels of IL-6 and IL-10, even in the early stages of the disease. Increased levels of IL-6 in patients with CKD have been found to be related with malnutrition [58], atherosclerosis and cardiovascular risk and all cause mortality [59,60]. IL-10 also is related with cardiovascular risk in ESRD [61], low levels of quality of life and nutritional status [62]. Moreover, as it has been described, cytokines and especially IL-6 release activate the JAK/STAT pathway which is involved in several experimental models and human renal diseases such as renal fibrosis. Both JAKs and STATs play an important role in the brain functions and especially STAT3 is implicated in brain pathologies such as brain tumors [63] and regulate and control the neuronal proliferation, survival and differentiation. Accordingly, in CKD patients the activation of the JAK/STAT pathway and especially the STAT3 stimulation due to IL-6 release, might be implicated in brain pathophysiology and accordingly in cognitive impairment that characterizes these patients.
In the study by Kaltsatou et al. [64], where the relation between inflammation and cognitive function was examined during a HD session, it was found that inflammation might contribute to cognitive impairment observed after the HD session. The fact that the inflammation status, which was measured by the index of CRP, worsened after the HD session, and a correlation between cognitive function and this index was observed, indicates that inflammation might influence cognitive function [64]. In this study CRP levels increased by 39.6% after the HD session [64]. CKD is considered an inflammatory process itself. Moreover, CKD abnormalities such as malnutrition, chronic volume overload and Autonomic Nervous System (ANS) dysfunction, contribute to increased inflammation. In addition, it has been suggested that cognitive impairment is related to the level of renal functional impairment and especially in ESRD, where the immune system is strongly activated, cognitive impairment is more profound. HD therapy has been reported to increase inflammatory biomarkers [65]. A possible explanation for this is that during the dialysis therapy, blood comes in contact with a membrane that allows bacterial products of low molecular weight, a passage into the patient’s organism. Moreover, endotoxin in dialysis fluid contributes to reactive oxygen species production by triggering the leukocytes [66]. Conversely, oxidative stress status, which is increased in HD patients [65], could promote the pro-inflammatory cytokine production by activating the nuclear factor NF-κB pathway [67]. In addition, loss of some antioxidants, such as carnitine, during the HD therapy may contribute to chronic inflammation that characterizes HD patients.
High levels of urea clearance during HD have been related to better cognitive function [7]. In addition, a study by Schneider et al. [68], who examined cognitive cognition 19 hours after the end of the HD therapy, found significant improvements in patient memory function. Specifically, after HD, patients had improvements in logical and visual long-term memories, processing speed and visual scanning, cognitive shifting and activity planning [68]. However, cognitive performance even after the HD therapy was impaired compared to the control group [68]. The results of this study indicated that cognitive impairment is reversible by HD and increasing the HD frequency could induce beneficial effects on patients’ cognitive function [68]. In the literature, there is an agreement that HD therapy improves cognitive function in CKD patients [69,70].
Although, it is well known that cognitive function is believed to be best 24 hours after the HD session, the variation of cognitive function during the HD circle has not been investigated yet. A study by Murray et al. [71], who evaluated cognitive performance one hour before the session, one hour into the session, and one hour after and the next day of the HD therapy, found that cognitive performance varies during the HD session. Specifically, the results of this study observed that cognitive performance was worse during the HD and better before the session and the day after, namely 24 hours after the HD treatment [71].
Importantly, there is evidence that there is an association between the ANS outflow and various inflammatory indices in patients with septic conditions or cardiac diseases [72,73]. Besides inflammation, there is evidence that ANS dysfunction is associated with mild cognitive function [74]. In patients with Alzheimer’s disease (AD) and Myasthenia Gravis the strong relationship between acetylcholine mediated neurotransmission and cognitive function has led to the development of the cholinergic hypothesis [75,76]. According to this, the degeneration of cholinergic neurons in the basal forebrain and the associated loss of cholinergic neurotransmission in the cerebral cortex and in other areas, contributed significantly to the deterioration in cognitive function, as observed in patients with AD [77,78] and Myasthenia Gravis [79]. ANS dysfunction, a common implication in CKD patients, is related to inflammation and cognitive impairment. In patients with CKD, clinical data show that altered cardiac autonomic tone remains one of the main reasons of the increased morbidity and mortality rates. Heart rate variability indices, which are accepted tools for the assessment of ANS activity, were decreased as a result of a sympathetic overestimation in HD patients [80].
Conclusions
To conclude, cognitive impairment is a common abnormality that characterizes CKD patients and is more profound in ESRD patients who receive the HD therapy. As long as the impact of inflammation on cognitive impairment in CKD patients, remains unknown, progress on this topic is slowed down. However, according to the results of these two studies that were found after the literature search in this systematic review, it can be concluded that inflammation might be implicated in cognitive impairment in CKD patients. However, further research should be done in this field in order to investigate the mechanisms that are implicated in cognitive impairment in CKD patients, and thus suggest new prevention strategies.
References
- Nicola LD, Zoccal Ci (2016) Chronic kidney disease prevalence in the general population: heterogeneity and concerns. Nephrol Dial Transplant 31: 331-335.
- Lozano R, Mohsen N, Kyle F, Stephen L, Kenji S, et al. (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095-2128.
- Kramer A, Pippias M, Stel VS, Bonthuis M, Abad Diez JM, et al. (2016) Renal replacement therapy in Europe: a summary of the 2013 ERA-EDTA Registry Annual Report with a focus on diabetes mellitus. Clin Kidney J 9: 457-69.
- Kurella M, Chertow GM, Luan J, Yaffe K (2004) Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 52: 1863-1869.
- Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, et al. (2006) Cognitive impairment in hemodialysis patients is common. Neurology 67: 216-223.
- Dahbour SS, Wahbeh AM, Hamdan M (2009) Mini mental status examination (MMSE) in stable chronic renal failure patients on hemodialysis: The effects of hemodialysis on the MMSE score. A prospective study. Hemodial Int 13: 80-85.
- Teschan PE, Bourne JR, Reed RB, Ward JW (1983) Electrophysiological and neurobehavioral responses to therapy: the National Cooperative Dialysis Study. Kidney Int Suppl 23: S58-S65.
- Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ (1997) Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis 30: 41-49.
- Mizumasa T, Hirakata H, Yoshimitsu T, Hirakata E, Kubo M, et al. (2004) Dialysis-related hypotension as a cause of progressive frontal lobe atrophy in chronic hemodialysis patients: a 3-year prospective study. Nephron Clin Pract 97: 23-30.
- Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, et al. (2007) Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab 27: 1861-1869.
- Beard CM, Kokmen E, O'Brien PC, Anía BJ, Melton LJ (1997) Risk of Alzheimer's disease among elderly patients with anemia: population-based investigations in Olmsted County, Minnesota. Ann Epidemiol 7: 219-224.
- Brines ML, Ghezzi P, Keenan S, Agnello D, Lanerolle NC, et al.(2000) Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A 97: 10526-10531.
- Chou FF (2008) Cognitive changes after parathyroidectomy in patients with secondary hyperparathyroidism. Surgery 143: 526-532.
- Goldstein DA (1980) The relationship between the abnormalities in electroencephalogram and blood levels of parathyroid hormone in dialysis patients. J Clin Endocrinol Metab 51: 130-134.
- Patel N, Dalal P, Panesar M (2008) Dialysis disequilibrium syndrome: a narrative review. Semin Dial 21: 493-498.
- Watanabe K., Watanabe T, Nakayama M (2014) Cerebro-renal interactions: impact of uremic toxins on cognitive function. Neurotoxicology 44: 184-193.
- Yeh YC, Huang MF, Liang SS, Hwang SJ, Tsai JC, et al. (2016) Indoxyl sulfate, not p-cresyl sulfate, is associated with cognitive impairment in early-stage chronic kidney disease. Neurotoxicology 53: 148-152.
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, et al. (1997) Aging, memory, and mild cognitive impairment. Int Psychogeriatr.9: 65-69.
- Trollor J, Agars E (2010) Systemic inflammation and cognition in the elderly., in Neuropsychiatric Disorders. Springer: Tokyo 177-198.
- Montinaro V, Iaffaldano GP, Granata S, Porcelli P, Todarello O, et al. (2010) Emotional symptoms, quality of life and cytokine profile in hemodialysis patients. Clin Nephrol 73: 36-43.
- Cunnigham C, Hennesy E (2015) Co-morbidity and systemic inflammation as drivers of cognitive decline: new experimental models adopting a broader paradigm in dementia research. Alzheime's Res Ther 7: 33.
- McGeer PL, McGeer E, Rogers J, Sibley J (1990) Anti-inflammatory drugs and Alzheimer disease. Lancet 335: 1037.
- McGeer PL, Schulzer M, McGeer EG (1996) Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer's disease: a review of 17 epidemiologic studies. Neurology 47: 425-432.
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, et al. (2005) Serum inflammatory proteins and cognitive decline in older persons. Neurology 64: 1371-1377.
- Holmes C, El-Okl M, Williams AL, Cunningham C, Wilcockson D, et al. (2003) Systemic infection, interleukin 1beta, and cognitive decline in Alzheimer's disease. J Neurol Neurosurg Psychiatry 74: 788-789.
- Jefferson AL, Massaro JM, Beiser AS, Seshadri S, Larson MG, et al. (2011) Inflammatory markers and neuropsychological functioning: the Framingham Heart Study. Neuroepidemiology 37: 21-30.
- Schram MT, Euser SM, de Craen AJ, Witteman JC, Frölich M, et al. (2007) Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc 55: 708-716.
- Economos A, Wright CB, Moon YP, Rundek T, Rabbani L, et al. (2013) Interleukin 6 plasma concentration associates with cognitive decline: the northern Manhattan study. Neuroepidemiology 40: 253-259.
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, et al. (2004) The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 292: 2237-2242.
- Trollor JN, Smith E, Baune BT, Kochan NA, Campbell L, et al. (2010) Systemic inflammation is associated with MCI and its subtypes: the Sydney Memory and Aging Study. Dement Geriatr Cogn Disord 30: 569-578.
- Rus HG, Vlaicu R, Niculescu F (1996) Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis 127: 263-271.
- Schieffer B, Schieffer E, Hilfiker-Kleiner D, Hilfiker A, Kovanen PT, et al. (2000) Expression of angiotensin II and interleukin 6 in human coronary atherosclerotic plaques: potential implications for inflammation and plaque instability. Circulation 101: 1372-1378.
- Ridker PM, Rifai N, Stampfer MJ, Hennekens CH (2000) Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 101: 1767-1772.
- Ridker PM, Hennekens CH, Buring JE, Rifai N (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342: 836-843.
- Ringheim GE, Burgher KL, Heroux JL (1995) Interleukin-6 mRNA expression by cortical neurons in culture: evidence for neuronal sources of interleukin-6 production in the brain. J Neuroimmunol 63: 113-123.
- Benveniste EN (1992) Inflammatory cytokines within the central nervous system: sources, function, and mechanism of action. Am J Physiol 263: C1-16.
- Woodroofe MN, Sarna GS, Wadhwa M, Hayes GM, Loughlin AJ, et al. (1991) Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J Neuroimmunol 33: 227-236.
- Hopkins SJ, Rothwell NJ (1995) Cytokines and the nervous system. I: Expression and recognition. Trends Neurosci 18: 83-88.
- Vallières L, Campbell IL, Gage FH, Sawchenko PE (2002) Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci 22: 486-492.
- Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302: 1760-1765.
- Drapeau E, Mayo W, Aurousseau C, Moal M, Piazza PV, et al. (2003) Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A 100: 14385-14390.
- Saxe MD, Malleret G, Vronskaya S, Mendez I, Garcia AD, et al. (2007) Paradoxical influence of hippocampal neurogenesis on working memory. Proc Natl Acad Sci U S A 104: 4642-4646.
- Hryniewicz A (2007) Impairment of recognition memory in interleukin-6 knock-out mice. Eur J Pharmacol 577: 219-220.
- Tso AR, Merino JG, Warach S (2007) Interleukin-6 174G/C polymorphism and ischemic stroke: a systematic review. Stroke 38: 3070-3075.
- Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, et al. (2004) Interleukin-6: a cytokine to forget. FASEB J 18: 1788-1790.
- McAfoose J, Baune BT (2009) Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev 33: 355-366.
- Yirmiya R, Goshen I (2011) Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun 25: 181-213.
- Seguin JA, Brennan J, Mangano E, Hayley S (2009) Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration. Neuropsychiatr Dis Treat 5: 5-14.
- Liu X, Wu Z, Hayashi Y, Nakanishi H (2012) Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience 216: 133-142.
- Thomson LM, Sutherland RJ (2005) Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res Bull 67: 24-29.
- Yadav A, Kalita A, Dhillon S, Banerjee K (2005) JAK/STAT3 pathway is involved in survival of neurons in response to insulin-like growth factor and negatively regulated by suppressor of cytokine signaling-3. J Biol Chem 280: 31830-31840.
- Darnell JE, Kerr IM, Stark GR (1994) Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264: 1415-1421.
- Darnell JE (1997) STATs and gene regulation. Science 277: 1630-1635.
- Levy DE, Darnell JE (2002) Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3: 651-662.
- Wang Y (2013) STAT3 activation in response to IL-6 is prolonged by the binding of IL-6 receptor to EGF receptor. Proc Natl Acad Sci U S A 110: 16975-16980.
- De-Fraja C (2000) STAT signalling in the mature and aging brain. Int J Dev Neurosci 18: 439-446.
- Van den Kommer TN (2010) Homocysteine and inflammation: predictors of cognitive decline in older persons? Neurobiol Aging 31: 1700-1709.
- Raj DS (2009) Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum 38: 382-388.
- Rao M (2005) Plasma interleukin-6 predicts cardiovascular mortality in hemodialysis patients. Am J Kidney Dis 45: 324-333.
- Kimmel PL (1998) Immunologic function and survival in hemodialysis patients. Kidney Int 54: 236-244.
- Girndt M (2002) Anti-inflammatory interleukin-10 genotype protects dialysis patients from cardiovascular events. Kidney Int 62: 949-955.
- Balakrishnan VS (2004) Cytokine gene polymorphisms in hemodialysis patients: association with comorbidity, functionality, and serum albumin. Kidney Int 65: 1449-1460.
- Cattaneo E (1998) Variations in the levels of the JAK/STAT and ShcA proteins in human brain tumors. Anticancer Res 18: 2381-2387.
- Kaltsatou A (2016) The Impact of Inflammation and Autonomic Nervous Ssystem Activity on Cognitive Impairment during a Hemodialysis Session. J Clin Exp Nephrol 1: 15.
- Spittle MA (2001) Oxidative stress and inflammation in hemodialysis patients. Am J Kidney Dis 38: 1408-1413.
- DeLeo FR (1998) Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J Clin Invest 101: 455-463.
- Lawrence T (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol 1: a001651.
- Schneider SM (2015) Effect of a single dialysis session on cognitive function in CKD5D patients: a prospective clinical study. Nephrol Dial Transplant 30: 1551-1559.
- Harciarek M (2009) Cognitive performance before and after kidney transplantation: a prospective controlled study of adequately dialyzed patients with end-stage renal disease. J Int Neuropsychol Soc 15: 684-694.
- Lux S (2010) Differential activation of memory-relevant brain regions during a dialysis cycle. Kidney Int 78: 794-802.
- Murray Am (2007) Acute variation in cognitive function in hemodialysis patients: a cohort study with repeated measures. Am J Kidney Dis 50: 270-278.
- Papaioannou VI, Pneumatikos, Maglaveras N (2013) Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: current strengths and limitations. Front Physiol 4: 174.
- Brunner EJ (2002) Adrenocortical, autonomic, and inflammatory causes of the metabolic syndrome: nested case-control study. Circulation 106: 2659-2665.
- Nicolini P (2014) Autonomic dysfunction in mild cognitive impairment: evidence from power spectral analysis of heart rate variability in a cross-sectional case-control study. PLoS One 9: e96656.
- Bartus RT (1982) The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408-414.
- Bartus RT (2000) On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol 163: 495-529.
- Contestabile A (2011) The history of the cholinergic hypothesis. Behav Brain Res 221: 334-340.
- Fotiou D (2015) Evaluation of the cholinergic hypothesis in Alzheimer's disease with neuropsychological methods. Aging Clin Exp Res 27: 727-733.
- Kaltsatou A (2015) Cognitive impairment as a central cholinergic deficit in patients with Myasthenia Gravis. BBA Clin 3: 299-303.
- Deligiannis A, Kouidi E, Tourkantonis A (1999) Effects of physical training on heart rate variability in patients on hemodialysis. Am J Cardiol 84: 197-202.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences