Strategies to Minimize Growth Retardation in Children with Steroid-Sensitive Nephrotic Syndrome
Alex R Constantinescu, Simona Maruntelu, Evelio Velis, Teofana O Bizerea-Moga, Manuela C Almaguer, Tamara M Marcovici, Alexandru B Constantinescu and Otilia Marginean
Alex R Constantinescu1,2,3*, Simona Marunţelu4,5, Evelio Velis6, Teofana O Bizerea-Moga4,5, Manuela C Almaguer2,7, Tamara M Marcovici4,5, Alexandru B Constantinescu8 and Otilia Mărginean4,5
1Department of Pediatric Nephrology, Joe DiMaggio Children’s Hospital, Hollywood, FL, USA
2Charles E. Schmidt College of Medicine at Florida Atlantic University, Boca Raton, FL, USA
3Kiran C. Patel College of Allopathic Medicine, Ft. Lauderdale, FL, USA
4Clinic 1 Pediatrics Emergency Hospital for Children “Louis Å¢urcanu”, Timişoara, Romania
5University of Medicine and Pharmacy, "Victor Babeş", Timişoara, Romania
6Barry University, Miami, FL, USA
7Department of Pediatric Endocrinology, Joe DiMaggio Children’s Hospital, Hollywood, FL, USA
8Lewis Katz School of Medicine, Temple University, Philadelphia, PA, USA
*Corresponding Author:
Alex R Constantinescu
MD, Chief, Pediatric Nephrology
Joe DiMaggio Children’s Hospital
1131 N35th Ave, Second Floor, Hollywood
FL 33021, USA
Tel: (+1) 954-265-9344
E-mail: aconstantinescu@mhs.net
Received date: March 14, 2016; Accepted date: April 19, 2016; Published date: April 22, 2016
Citation: Constantinescu AR, Marunţelu S, Velis E, Bizerea-Moga TO, Almaguer MC, et al. (2019) Strategies to Minimize Growth Retardation in Children with Steroid-Sensitive Nephrotic Syndrome. J Clin Exp Nephrol Vol.4 No.2: 76.
Copyright: © 2019 Constantinescu AR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Growth suppression is a known complication of prolonged steroid therapy in children with nephrotic syndrome (NS). Those with frequent relapses (FR) and those steroid-dependent (SD) have a significant growth delay compared to children with infrequent relapses (IR). There is substantial variability in the way childhood NS is treated and the majority of these children will exhibit improved growth rate after cessation of therapy, though some will not undergo catch-up growth, experiencing issues related to short stature later in life. The aims of our study were to assess growth deficits in children with FR/SD NS and evaluate the impact and the appropriate time to introduce a steroid-sparing agent (SSA).
Methods and findings: We are presenting the growth data in pre-pubertal children (<10 years of age) followed in two geographically distinct pediatric centers, and discuss approaches to reduce the negative impact of steroids on growth. The main data collected were growth rate (GR) and height standard deviation score (HtSDS). Statistical analysis was performed using ANOVA, regression analysis and student t-test.
The GR (in cm/yr) was 6.94 ± 4.99 and 10.2 ± 5.4 in IR, and 5.86 ± 5.06 and 4.36 ± 2.45 in FR/SD, at 6 and 12 months after diagnosis, respectively. The GR was slower in the second 6 months period in FR/SD (n=9) compared to IR (n=16): 4.4 ± 2.5 vs. 10.2 ± 5.4 cm/yr (p<0.001). The HtSDS change, independent of location, was more pronounced in FR/SD:-0.53 ± 0.47 compared to 0.16 ± 0.53 in patients with IR, p=0.003. Previous reports of early detrimental impact of steroids on growth have suggested the possibility of a positive effect of therapy with a SSA. We have retrospectively looked at 10 patients with FR/SD disease course whose therapy was switched from steroids to SSA, and HtSDS improved from -1.12 ± 1.23 to -0.73 ± 1.21, p<0.001. We discuss additional facts about steroid therapy in NS and strategies to limit the patients’ exposure to them.
Conclusion: We conclude that strategies to minimize steroid exposure, such as reducing the duration of therapy based on response time and the use of SSA can prevent the growth deficit seen in children with FR/SD NS.
Keywords
Nephrotic syndrome; Growth suppression; Steroid therapy; Glomerular membrane permeability
Introduction
Nephrotic syndrome (NS) was defined in 1963 by Schreiner as a clinical entity having multiple causes, characterized by high glomerular membrane permeability, manifested by massive proteinuria and lipiduria, in the absence of depressed glomerular filtration rate (GFR) [1].
Quantifying protein excretion is essential in differentiating nephrotic from non-nephrotic range proteinuria, since dipstick analysis is only qualitative. Nephrotic range proteinuria is defined in children when urine protein excretion is above 40 mg/m2/hr in a timed specimen, or above 1 g protein/g creatinine in a random sample, preferably first morning urine specimen [2].
International Study of Kidney Diseases in Children (ISKDC) [3] characterized idiopathic NS in children based on the kidney biopsy findings, and identified the preponderance of minimal change disease (MCD) at early age. Based on these findings, steroid therapy is initiated first, with biopsy being considered in selected cases. This is in contrast to adult patients, who undergo a kidney biopsy first, followed by specific treatment based on pathology findings [4].
Protocols for initial therapy vary widely, from ISKDC proposed 8 weeks (4 weeks of daily 60 mg/m2 followed by 4 weeks of alternate daily therapy 40 mg/m2 per dose) [3,4], to 12 weeks of steroids (6 weeks of daily 60 mg/m2 and 6 weeks of alternate daily 40 mg/m2 per dose) [5] without further gains by extending the initial treatment duration to 6 months [6].
Based on response (defined as protein-free urine on dipstick analysis), patients are classified as having steroid-sensitive (SSNS) or steroid-resistant (without response after 4-6 weeks of therapy). NS relapses are defined as follows: infrequent (IR) if less than 2 relapses in a year, frequent (FR) if 2 relapses in 6 months, or more than 3 relapses in a year, and patients can have a steroid-dependent (SD) course if a relapse occurs less than a month after completion of steroid therapy, or while patient is receiving alternate daily therapy.
Aside from early gastro-intestinal, cardiac, ocular and metabolic side effects of steroids, body image, psychosocial adaptation, bone metabolism, and pubertal development are significant long-term consequences of steroid therapy, along with the impact on growth.
In 1968, Lam et al. [7] demonstrated that steroids affect linear growth. Their effects at central and peripheral sites were later described: centrally, by stimulating the somatostatin they inhibit the pulsatile growth hormone (GH) secretion, and peripherally, they decrease the GH receptor expression and binding, as well as insulin-like growth factor-1 (IGF-1) activity. These, in addition to the effects on bone mineralization and the fact that these children are exposed to steroids at an early stage in their bone development process, have a cumulative negative impact on the growth plate, leading to short stature.
Aim of the Study
The aims of the study were to analyze the effects of steroids on the growth patterns of pediatric patients with SSNS and evaluate the impact of strategies targeting the minimization of steroid exposure on growth.
Materials and Methods
Study design
The multicenter retrospective study was conducted over a period of 5 years, at “Louis Å¢urcanu” Children’s Clinical and Emergency Hospital, Timişoara, Romania and at Joe DiMaggio Children’s Hospital, Hollywood FL, USA.
Patient selection
Patient selection was based on a single exclusion criterion: glomerular diseases other than MCD. The study was approved by the ethics committee/institutional review board of both hospitals where the study was conducted. Patient information was de-identified as per institutions’ protocol.
Therapy protocols
Two cohorts of pre-pubertal children (≤ 10 years of age) with SSNS, the majority treated based on modified ISKDC protocol, 4-6 weeks daily and 4-6 weeks alternate daily dosing, followed for at least a year, were studied in two different settings (Romania-Group 1 and USA-Group 2).
Based on our initial clinical observations [8], we noticed that the response time was a determinant factor in the rate of relapse: those who had a rapid response had a significantly higher probability of having IR. Duration of therapy was determined by the response time. If hematuria was present and/or the response was seen after 7 days, patients received 12 weeks of therapy (“6+6”). On the other hand, if patients did not have hematuria and the response was seen in the first week, the therapy was shortened to 8 weeks (“4+4”).
In some patients with FR/SD disease course, therapy was changed to a steroid-sparing agent (SSA) and growth rate (GR)/ height standard deviation score (HtSDS) were compared to those seen in patients with IR disease course.
Data collection
The following data were gathered: age, gender, race, standing height at onset of the disease, then at 6 months, 1 year and at last visit, when available. Height was measured using the Harpenden Stadiometer in Romania and at JDCH by using a stadiometer manufactured by Holtain Limited, UK. The mean value of three separate measurements was calculated and each recorded data set was compared to height for age, using the WHO Growth charts. Additional variables recorded were: relapse pattern, GR and HtSDS at 6 and 12 months, along with HtSDS at last visit.
Statistical analysis section
The Statistical Package for Social Sciences (SPSS 26®) was used to organize, validate and analyze collected data. Indicators of central tendency and dispersion were calculated. Student's t-tests and Analysis of Variance (ANOVA) were performed to detect significant differences between selected groups. Unadjusted linear regression was used to evaluate prediction between growth rate (GR) and height standard deviation (HtSDS) scores. A determining level of 0.05 was selected for all as test of significance.
Results
There were 29 pre-pubertal children with SSNS (mean age: 3.9 ± 2.4 yr-group 1, and 4.3 ± 1.5 yr-group 2) with complete data at 12 months, and 25 with complete data at 6 mos. There were 14 children in group 1 and 15 in group 2, 20 with IR, 9 with FR/SD disease course, 16 were Caucasians, 9 were African-Americans and 4 were Hispanics. Characteristics of these cohorts can be seen in Table 1. The GR and HtSDS values can be seen in Table 2.
TSRAge at dx
(yrs)GenderRelapse
patternID number
JDCHAge at dx
(yrs)GenderRelapse
patternRace ethnicity13FFR14FSD 22.5FFR25MIRAA33.17FIR33FIRAA43MIR46.7MIRAA51.33FIR52.5MFRH67MSD63.7FIRAA73.7MIR72.2FFRAA81.4MFR84.5MIRAA91.6FIR94.8FIRH1010MIR103.5MIRAA112.5MIR117FIRH124.4FIR125.5MFRAA134.8MIR133.4FIRH146MIR146.3FSD 153FIRAA
Table 1: Patient characteristics. TSR-patients in Group 1, JDCH-patients in Group 2. Relapse pattern-IR: Infrequent Relapses; FR: Frequent Relapses; SD: Steroid-Dependent; Race/Ethnicity-AA: African-Americans; H: Hispanics; all others Caucasians. All patients received steroids for initial therapy, not less than 8 weeks and not more than 12 weeks, as well as for treatment of relapses.
Table 2: Growth Rates (GR) in cm/yr and height standard deviation score (HtSDS) values at the end of the two time periods (0-6 and 6-12 months after therapy) along with HtSDS change (ΔHtSDS) in both groups of patients, depending on the disease course: Infrequent Relapses (IR) or Frequent Relapses/Steroid-Dependent (FR/SD). Group 1-Romania, Group 2-USA.
Patients with FR/SD from both study sites exhibited a slower GR in the second six months period of the first year after diagnosis, from 9.14 ± 4.92 to 6.01 ± 2.77 cm/yr in group 1, and from 8.61 ± 4.91 to 6.55 ± 2.71 cm/yr in group 2. Patients with IR from both study sites exhibited catch-up growth based on GR measured at 6 and 12 months following diagnosis, from 6.94 ± 4.99 cm/yr in the first 6 months, to 10.2 ± 5.4 cm/yr in the second 6-month period (p=0.045), compared to patients with FR/SD disease course who appeared to experience a growth deceleration, from 5.86 ± 5.06 to 4.36 ± 2.45 cm/yr in the same time frame, even though it did not reach statistical significance (p=0.08). During the second 6-month period, GR was slower in FR/SD (n=9) compared to IR (n=16):4.4 ± 2.5 vs. 10.2 ± 5.4 cm/yr (p<0.001).
During the first 6 months following diagnosis, the HtSDS change (ΔHtSDS) was 0.06 ± 0.4 for IR group (n=16) and -0.61 ± 0.66 for FR/SD group (n=9) (p=0.009). The GR at 6 months is a significant predictor of ΔHtSDS at 12 months, p=0.048, sixteen percent (R2=0.16) of the variation in ΔHtSDS being explained by variation in this GR. As for the second 6-month period, the ΔHtSDS was 0.16 ± 0.53 for the IR group and -0.53 ± 0.47 for the FR/SD group (p=0.003). The GR at 12 months is a significant predictor of ΔHtSDS, p=0.02, around eighteen percent (R2=0.183) of the variation in ΔHtSDS being explained by variation in GR at 12 months (Figure 1). There were no geographical or racial differences.
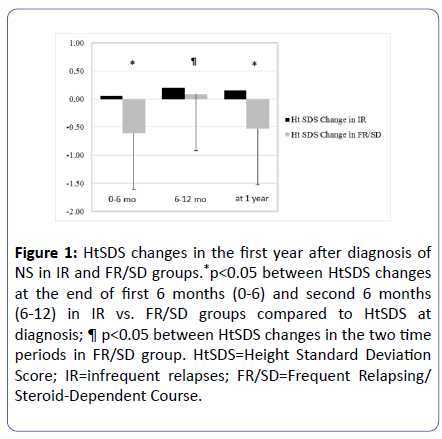
Figure 1:HtSDS changes in the first year after diagnosis of NS in IR and FR/SD groups.*p<0.05 between HtSDS changes at the end of first 6 months (0-6) and second 6 months (6-12) in IR vs. FR/SD groups compared to HtSDS at diagnosis; ¶ p<0.05 between HtSDS changes in the two time periods in FR/SD group. HtSDS=Height Standard Deviation Score; IR=infrequent relapses; FR/SD=Frequent Relapsing/ Steroid-Dependent Course.
The results so far demonstrate that children with FR/SD disease course experience a reduction in growth velocity as early as 6 months, without catch-up growth at 12 months. There is a significant inverse (negative) relationship between relapsing pattern (FR or SD) and GR measured at 12 months, F(1,27)=9.91, p=0.004. Relapsing pattern also explained a significant proportion of variation in GR, R2 of 0.27, with the difference in the average GR being significantly higher among IR patients than FR/SD patients, t(27)=-3.15 (p=0.004).
The new electronic charting (Epic, Verona, WI 53593, USA), has helped us track the new patients, and we reviewed the charts on our active patients with at least one year of followup after the switch to a SSA.
The relapse pattern, initial steroid course and HtSDS at the last visit were studied in 26 children followed for at least one year after diagnosis [9]. IR disease course was seen in 62% of these children, and a third of them received a total of 8 weeks of steroids, since they did not have hematuria at presentation and became protein-free within the first week of treatment. An analysis of their growth pattern revealed that the GR of the IR patients treated for 8 weeks was better than that of IR patients treated for 12 weeks, with both groups having a GR significantly better than the children with FR/SD disease course. Reassuring was the fact that after replacing steroids with SSA, FR/SD patients had a GR closer to that of IR patients treated for 12 weeks [9], reducing the height deficit. There was a significant improvement in HtSDS after the therapy change in 10 patients: from -1.12 ± 1.23 to -0.73 ± 1.21 (paired t-test with p<0.001 between pre-SSA and last visit on SSA), and the statistical significance was lost when HtSDS of those patients who received SSA was compared to HtSDS of children who had IR disease course and were treated with steroids for a total of 8 weeks (n=5) or 12 weeks (n=10) (p=0.29 and p=0.25, respectively) [9].
Discussion
Our study has validated some of the previous findings and added information about the catch-up growth seen in patients exposed to a smaller dose of steroids, who benefited from SSA, suggesting that there can be a positive effect on growth in patients receiving new therapies such as rituximab, mycophenolate mofetil or calcineurin inhibitors, early on.
As pointed out in previous studies, the growth delay was more pronounced in children with FR/SD disease course compared to the children exhibiting IR disease course [10].
Even though 40% of children will experience no relapses or will have IR disease course [9], a few possibilities to minimize the exposure to steroids can be contemplated: decreasing the frequency of relapses, use of a lower steroid dose initially, use of an alternate therapy altogether, or use the response time to tailor the therapy.
A few additional questions have been posed over the years: What affects growth rate-renal pathology or steroid therapy? When is the maximal impact of steroid therapy on growth rate noted? Does geographical location or racial heterogeneity play a role in relapse pattern or growth delay? Do steroid-sparing treatment strategies have a beneficial effect of growth rate? What is the best timing for consideration of a SSA? [9]
Can relapses be predicted?
A single center retrospective analysis of children with NS [8], was carried out to identify what symptom, or laboratory finding, if any, at the time of presentation, could help predict the relapse pattern. There were complete records for 56 children, average age of 4 years, 68% boys. At that time, treatment for the most part followed the ISKDC protocol, total of 8 weeks of therapy. As can be seen in Figure 2, patients with a faster response to therapy (within 7 days after initiation of steroid therapy), who did not exhibit hematuria at presentation, were predicted to have an IR disease course, with a positive predictive value of 94%.
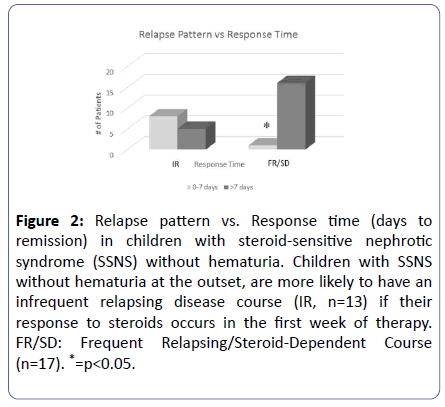
Figure 2:Relapse pattern vs. Response time (days to remission) in children with steroid-sensitive nephrotic syndrome (SSNS) without hematuria. Children with SSNS without hematuria at the outset, are more likely to have an infrequent relapsing disease course (IR, n=13) if their response to steroids occurs in the first week of therapy. FR/SD: Frequent Relapsing/Steroid-Dependent Course (n=17). *=p<0.05.
A few years later, Wasilewska et al. [11] studied the multidrug resistance (MDR1) gene polymorphisms in children with NS. The MDR1 gene encodes for p-glycoprotein, an active transmembrane efflux pump, and the poor response to steroid therapy in some patients can be explained by MDR1 gene upregulation. However, the genetic testing is not widely available to predict who will have a prompt or a delayed response to steroids. Our clinical observation could not predict the response to steroids, but the response time was a determinant factor in the rate of relapse. Those with a rapid response had a significantly higher probability of having IR, opposite to the TT genotype of the MDR-1 gene.
Subsequent studies revealed similar data around the world. Mishra et al., [12] identified younger age and longer time to remission as predictors of FR course. Harambat et al., [13] found that among FR/SD children, longer time to remission predicted use of SSA. Sureshkumar et al., [14] from Australia, have published their prospective analysis of 129 children with SSNS, and identified male gender, young age and short time to first relapse as predictors of a FR disease course.
What does affect the growth rate-renal pathology or steroid therapy?
Glomerular diseases other than MCD, presenting with nephrotic syndrome, likely due to the required higher doses of steroids for a longer time, as well as the associated decline in renal function, can lead to short stature. Over a period of 2 years, 11 consecutive children with NS, having various glomerular diseases other than MCD, were compared to 9 children with MCD. The HtSDS at the last follow-up was lower in children with glomerular diseases other than MCD, -0.97 ± 0.94 compared to 0.14 ± 0.91 in children with MCD (p=0.016) (A.B.C.-unpublished observation). This can be interpreted as catch-up growth in children with MCD as well as significant impact of non-MCD on final height.
Since non-MCD pathology causes a significant growth delay, independent of steroid therapy, subsequent studies excluded patients with steroid-resistant NS and those with biopsyproven pathology other than MCD.
When is the maximal impact of steroid therapy on growth rate?
Spreafico et al. [15] described the detrimental effects of steroids on osteoblastogenesis and apoptosis of osteocytes, with resulting stunted growth. However, as mentioned above, steroids remain the first-line of therapy in children with MCD, at a time when their bones are most vulnerable.
To study the issue in more detail, a retrospective analysis identified the degree of growth suppression, the “moment of maximum impact”, its prevalence and duration, with the goal of identifying catch-up growth [16]. In that study a database was created, excluding the patients with glomerular diseases other than MCD, and plotted HtSDS as well as growth rate standard deviation score (GRSDS) over the observation period. Records from 69 children were analyzed, and HtSDS was found to be below -1.8 in about 10% of patients. HtSDS values at 3 months, 6 months and one year, showed that the most growth suppression occurs in the first 3 months of therapy, a finding supported by a lower GR at 3 months. An additional observation was the lack of increase in GR between the follow up visits at 6 and 12 months in patients with FR/SD NS, the most likely reason why patients were falling below -2 SD in later years. It is also possible that the cumulative dose of steroids needed to treat relapses has an additive effect on growth suppression, placing the patients with FR/SD disease course at a higher risk. In support of these findings is the study by Emma et al., [17] who found the average height loss to be up to 1.8 SD below the mean in children with FR/SD NS. This correlated with steroid dose and was more prevalent in younger children.
In children with NS, growth suppression is maximal in the first 3 months after diagnosis and is commensurate with the duration of therapy and frequency of relapses [16,17].
Does geographical location or racial heterogeneity play a role in relapse pattern or growth delay?
This was one of the aims of our study and for both pubertal development and psychosocial adaptation to have a normal course, prediction of long term growth suppression is desired. As pointed out in this study and in previous ones, the growth delay is more pronounced in children with FR/SD disease course compared to the children exhibiting IR disease course [10]. In addition, we did not find any geographical or racial differences, albeit our sample was somewhat small.
Do steroid-sparing treatment strategies have a beneficial effect on growth rate, and what is the best timing for consideration of a steroidsparing agent?
It has been shown [5] that a longer initial steroid therapy course decreased the relapse rate, but it did lead to a higher cumulative steroid dose. Cyclosporine was introduced in the treatment of NS as a SSA, but aside from not providing a sustained remission, it did bring a new set of side effects. Overtime, recommendations have been made to add a calcineurin inhibitor (CNI) at the outset, though CNI toxicity has prevented the wide-spread adoption of this approach [18,19].
Following our initial observations, supported over the years by other investigators, children with NS were treated based on initial response time and some of those with FR/SD course have been placed on SSA (mostly tacrolimus). Despite the small sample, at the last follow-up visit, the HtSDS of these children was significantly better than HtSDS prior to initiation of SSA therapy (Figure 3).
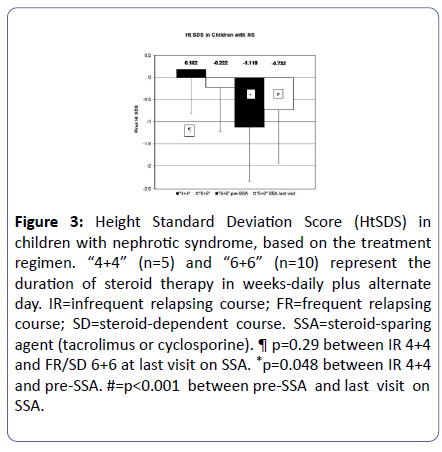
Figure 3: Height Standard Deviation Score (HtSDS) in children with nephrotic syndrome, based on the treatment regimen. “4+4” (n=5) and “6+6” (n=10) represent the duration of steroid therapy in weeks-daily plus alternate day. IR=infrequent relapsing course; FR=frequent relapsing course; SD=steroid-dependent course. SSA=steroid-sparing agent (tacrolimus or cyclosporine). ¶ p=0.29 between IR 4+4 and FR/SD 6+6 at last visit on SSA. *p=0.048 between IR 4+4 and pre-SSA. #=p<0.001 between pre-SSA and last visit on SSA.
Conclusion
We can surmise that since steroids have growth– suppression potential, independent of geographical location or race, attempts need to be made to minimize exposure to them. Cumulative dose can be decreased by predicting IR pattern based on response within one week and, in some centers, along with the absence of hematuria. The difference in GR tends to be noted in the first 6 months, but reaches significance 12 months after initial steroid course [10]. Therefore, the use of SSA needs to be considered if by one year after diagnosis, or even sooner, catch-up growth is not seen in patients with FR/SD SSNS. Standardized treatments could help understand the impact of growth suppression on the final target height, and develop a set of growth markers that may be unique to children with NS, based on the relapse pattern, including the calculation of mid-parental height for assessing the growth deficit early in the course of their disease.
Our studies, despite small sample sizes, represent only a few possible ways of minimizing the impact of steroids on growth suppression, as the quest for inducing lasting remission and reducing the number of relapses continues [20].
Acknowledgments
We would like to thank our office staff for their patience and attention to detail in measuring these children with utmost precision.
References
- Schreiner GE (1963) The nephrotic syndrome in Diseases of the Kidney. 80: 335-444.
- Hogg RJ, Portman RJ, Milliner D, Lemley KV, Eddy A, et al. (2000) Evaluation and management of proteinuria and nephrotic syndrome in children: Recommendations from a pediatric nephrology panel established at the National Kidney Foundation conference on proteinuria, albuminuria, risk, assessment, detection, and elimination (PARADE). Pediatrics 105: 1242-1249.
- No authors listed (1981) The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561-564.
- Tarshish PE, Tobin JN, Bernstein J, Edelmann CM (1997) Prognostic significance of the early course of minimal change nephrotic syndrome: Report of the International Study of Kidney Disease in Children. Clin J Am Soc Nephrol 8: 769-776.
- Brodehl J, Ehrich JHH (1988) Arbeitsgemeinschaft für Pädiatrische Nephrologie. Short vs. standard prednisone therapy for initial treatment of idiopathic nephrotic syndrome in children. Lancet 1: 380-383.
- Teeninga N, Kist-van Holthe JE, van Rijswijk N, Nienke I, Hop WC, et al. (2013) Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. Clin J Am Soc Nephrol 24: 149-159.
- Lam CN, Arneuil GC (1968) Long-term dwarfing effects of corticosteroid treatment in childhood nephrosis. Arch Dis Child 43: 589-594.
- Constantinescu AR, Shah HB, Foote EF, Weiss LS (2000) Predicting first-year relapses in children with nephrotic syndrome. Pediatrics 105: 492-495.
- Constantinescu A (2014) Strategies to minimize growth retardation in children with steroid-sensitive nephrotic syndrome. J Nephrol Ther 4: 75.
- Marginean O, Constantinescu AR (2016) Growth suppression in children with frequently-relapsing/steroid-dependent nephrotic syndrome. Pediatr Nephrol 31: 1845.
- Wasilewska A, Zalewski G, Chyczewski L, Zoch-Zwierz W (2007) MDR-1 gene polymorphisms and clinical course of steroid-responsive nephrotic syndrome in children. Pediatr Nephrol 22: 44-51.
- Mishra OP, Abhinay A, Mishra RN, Prasad R, Pohl M (2013) Can we predict relapses in children with idiopathic steroid-sensitive nephrotic syndrome? J Trop Pediatr 59: 343-349.
- Harambat J, Godron A, Ernould S, Rigothier C, Llanas B, et al. (2013) Prediction of steroid-sparing agent use in childhood idiopathic nephrotic syndrome. Pediatr Nephrol 28: 631-638.
- Sureshkumar P, Hodson EM, Willis NS, Barzi F, Craig JC (2014) Predictors of remission and relapse in idiopathic nephrotic syndrome: A prospective cohort study. Pediatr Nephrol 29: 1039-1046.
- Spreafico A, Frediani B, Capperucci C, Leonini A, Gambera D, et al. (2006) Osteogenic growth peptide effects on primary human osteoblast cultures: Potential relevance for the treatment of glucocorticoidâ€ÂÂÃâÃÂinduced osteoporosis. J Cell Biochem 98: 1007-1020.
- Cederbaum N, Constantinescu A. (2002) Steroid-induced growth suppression in children with nephrotic syndrome. J Investig Med 50:187.
- Emma F, Sesto A, Rizzoni G (2003) Long-term linear growth of children with severe steroid-responsive nephrotic syndrome. Pediatr Nephrol 18: 783-788
- Niaudet P, Habib R (1994) Cyclosporine in the treatment of idiopathic nephrosis. J Am Soc Nephrol 5: 1049-1056.
- Hoyer PF, Brodeh J (2006) Initial treatment of idiopathic nephrotic syndrome in children: prednisone vs. prednisone plus cyclosporine A: A prospective, randomized trial. J Am Soc Nephrol 17: 1151.
- Pal A, Kaskel F (2016) History of nephrotic syndrome and evolution of its treatment. Front Pediatr 4: 56.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences
