Sodium-Glucose Transporter Inhibitors and Diabetic Nephropathy in Humans and Animal Model
Nakhoul Rola, Koch Elias, Nakhoul Farid, Dahan Inbal, Evgeny Farber, Hanut Anam, and Nakhoul Nakhoul
Published Date: 2018-05-24DOI10.21767/2472-5056.100061
Nakhoul Rola1, Koch Elias2, Nakhoul Farid3,4,5*, Dahan Inbal4, Evgeny Farber3, Hanut Anam3 and Nakhoul Nakhoul3
1Department of Medicine, University of Szeged, Szeged, Hungary
2Department of Medicine, Friedrich-Alexander-Universität, Erlangen-Nürnberg Schlossplatz, Erlangen, Germany
3Department of Nephrology and Hypertension, Baruch Padeh, Poriya Medical Centre, Israel
4Diabetes and Metabolism Lab, Baruch Padeh Poriya Medical Centre, Israel
5Department of Medicine, Bar Ilan University, Ramat Gan, Israel
- *Corresponding Author:
- Nakhoul Farid
Department of Nephrology and Hypertension
Baruch Padeh, Poriya Medical Centre, Israel
Tel: +9724-665-2587
E-mail: fnakhoul@poria.health.gov.il
Received date: April 27, 2018; Accepted date: May 16, 2018; Published date: May 24, 2018
Citation: Rola N, Elias K, Farid N, Inbal D, Farber E, et al. (2018) Sodium-Glucose Transporter Inhibitors and Diabetic Nephropathy in Humans and Animal Model. J Clin Exp Nephrol Vol 3:10. doi: 10.21767/2472-5056.100061
Copyright: © 2018 Rola N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Diabetic nephropathy as the leading cause for end stage renal disease and replacement therapy is increasing every year. Treatment of T2DM with the present oral blood glucose lowering drugs and insulin is challenging, with an enormous number of patients are able to achieve the target glycaemic control (HbA1C<6.5%). Despite the use of new Insulin compounds and different recommended combination of oral anti-diabetic drugs, the benefits of these recommendations are offset by side effects such as weight gain and recurrent hypoglycaemia. Therefore, the need for new agents that control blood glucose strictly and have other proactive cellular pathways is challenging. The sodium glucose transporter protein 2 (SGLT2) inhibitors, are recently being widely used.
The main therapeutic effect of these new drugs, SGLT2 inhibitors (SGLT2-I), is lowering the blood glucose levels via inhibitory effect on the transport of glucose and sodium in the proximal tubular cells by sodium glucose transport 1. SGLT2-I reduce plasma sodium level by natriuretic and diuresis, with decreasing blood pressure and body weight. These new medications can be used as first and second lines of treatment especially in patients with normal glomerular filtration rate, with or without cardiovascular complications. The most effective combination of SGLT2I is Metformin especially in albuminuria and slowing the progression of diabetic nephropathy especially if initiated in early stages of DM. The new class of medication (SGLT2I) are less effective in patients with moderate CKD (eGFR<45 ml/min). This review will focus on the new pathways such autophagy as a new pathway where SGLT2 are involved with protective effects.
Keywords
Diabetes mellitus; Glomerular filtration rate; Diabetic kidney disease; Chronic kidney disease; End stage renal disease; Sodium glucose transporter protein 2 inhibitors; Autophagy
Abbreviations
DKD: Diabetic Kidney Disease, DN: Diabetic Nephropathy, ESRD: End Stage Renal Disease, mTORC1: Mammalian Target of Rapamycin Complex 1, RAAS: Renin-Angiotensin-Aldosterone- System, SGL2I: Sodium Glucose Transporter Inhibitors, T2 DM: Type 2 Diabetes Mellitus, MR: Magnetic Resonance
Introduction
Diabetic nephropathy is the leading cause of end stage renal failure worldwide. And at present it accounts for approximately 50% of all ESRD patients on replacement therapy [1,2]. The most known risk factors can be classified as susceptibility factors such as sex and genetics (es. Haptoglobin genotype) and initiation factors for DN such as hyperglycaemia, and progressive factors such as uncontrolled glucose level and arterial hypertension [3]. The main risk factor for DKD is the glucose cell toxicity [4,5]. Strict blood glucose control early in the course of the disease exerts a long-lasting favourable effect on the risk of DN development. This “legacy effect”, also named “metabolic memory”, suggests that early intensive glycaemic control can prevent irreversible renal parenchymal damage and replacement therapy [6].
The natural history of diabetic nephropathy includes different stages: Glomerular hyper filtration, micro albuminuria with incipient diabetic nephropathy, and diabetic nephropathy until irreversible renal failure. The early stages almost reversible with blood glucose control. High glucose is associated with podocytes and PCT cells damage (Glomerular and tubular damage) with consequent glomerular hypertrophy, mesangial expansion and sclerosis. The uses of converting enzyme inhibitor, angiotensin receptor blocker alone or in combination with aldosterone blockers are insufficient in the early stages of diabetic nephropathy. Despite the RAAS and combined anti hyperglycaemic drugs, still there is a risk for the progression of diabetic nephropathy [7-10]. The clinical diagnosis of diabetic nephropathy is based on glomerular filtration rate (eGFR) and albuminuria along with clinical features, such as the hyperglycaemic state duration, proliferative retinopathy or other cardiovascular complications. However, intensive glucose and blood pressure control after onset of proteinuria and plasma creatinine elevation has not been shown to reduce risk of diabetic nephropathy progression [5,6].
Many markers have been used to predict early-stage renal dysfunction in diabetic patients. Neutrophil gelatinise-associated lipocalin (NGAL) and serum cystatin C (CysC) as markers of renal dysfunction in T2D. The presence of micro-albuminuria is a sign of the presence of diabetic kidney disease and marks the need for more intense glucose and blood pressure control. Diffusionweighted MR imaging is an imaging modality that utilizes diffusion of water to characterize the structural changes of the tissue (Diffusion tensor imaging). This method is in use in assessment of normal renal parenchyma, renal fibrosis, and diabetic nephropathy in human in some centres in the world [11,12].
Once the irreversible state characterized with increased excretion of protein and plasma creatinine elevation is established, it is only possible to slow the progression of DN toward ESRD. Until recently, treatments with drugs that block the renin-angiotensin-aldosterone system (RAAS) have a proven beneficial effect in slowing kidney disease progression among those with diabetes; but their benefit is limited and they do not stop disease progression. Despite aggressive use of dual blockade of the renin-angiotensin-aldosterone system, and combination anti-diabetic therapy, the number of patients with diabetis mellitus who progress to end stage renal disease and replacement therapy is increasing [2,13,14].
Recently, new sodium-glucose transporters-2 inhibitors have become available: (Canagliflozin, Dapagliflozin, and Empagliflozin). This new drugs belong to the latest class of anti-diabetic agents to be introduced as first or second line treatment of diabetes mellitus type II. This decrease reabsorption of glucose to the PCT cells , which is enhanced in individuals with type 2 diabetes mellitus (T2DM), contributes to maintain near normal blood glucose levels, decrease glomerular hyperfiltration via tubuloglomerular feedback (TGF). Impressive the data in the literature showing the dual protective effect of SGLT2-I in improving glycaemic control in T2DM, and also through activation of the autophagic process [14-17].
Pathogenesis
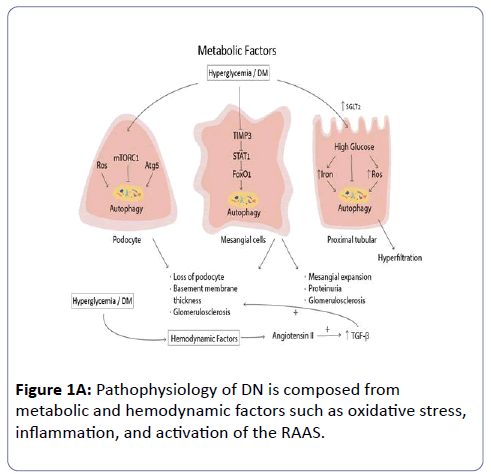
Regarding the complicated pathogenesis of DN, there are several separate stages, including: reversible glomerular hyperfiltration, normal glomerular filtration, normoalbuminuria, normal or decreasing glomerular filtration and micro-albuminuria, declining glomerular filtration and macroalbuminuria, until proteinuria with ESRD [17,18]. The pathophysiology of DN is composed from metabolic and hemodynamic factors such as oxidative stress, inflammation, and activation of the RAAS (Figure 1A). These activate inflammatory mediators and ischemic and fibrotic pathways leading to mesangial matrix accumulation, podocytes loss, glomerular basement membrane (GBM) thickening, tubular atrophy, tubulointerstitial inflammation, and fibrosis. Hyperglycaemia mediated alteration in extra- and intra-cellular metabolism including advanced glycation end products (AGEs), the enhancement of diacylglycerol/protein kinase C (DAG/PKC) activity, and increased flux through the polyol and hexosamine pathways constitute the main classical pathways involved in the pathogenesis of diabetic nephropathy [18-20]. Recently, the well-known autophagic process had been supposed as the main driven pathway involved in the evolution and generation of DN. Down regulation of the basal autophagic process activity in the podocytes and PCT cells, via the mTORC1 pathway. Autophagy, a lysosomal degradation pathway, plays a crucial role in cleaning protein aggregates and damaged organelles, including mitochondria, to maintain intracellular homeostasis. Therefore, autophagy may promote cellular health against various stress conditions, including endoplasmic reticulum (ER) stress. Increased cellular stresses induced by metabolic dysfunction, which is often observed in hyperglycaemia, impairs autophagy through activation of mammalian target of rapamycin complex 1 (mTORC1) and the reduction of AMP-activated kinase (AMPK) and Sirt1 activity [21-23].
Different studies hypothesize that up-regulation of sodium glucose co-transporter 2 (SGLT2) expressions due to hyperglycaemia, result in functional and structural changes including down regulation of the autophagy pathway in podocytes and PCT cells. So the new class of drugs including the SGLT2I can be of maggior benefit in slowing the progression of DN especially in early stages.
Mechanism of action of SGLT2 inhibitors
SGLT2-I are glucose-lowering agents that reduce the reabsorption of glucose and promote glucose excretion in the urine, thereby reducing hyperglycaemia in patients with T2DM [24]. Three SGLT2-I–Canagliflozin, Dapagliflozin, and Empaglifloxin-have been approved for the treatment of T2DM in the United States and Europe. Effectively, all of the glucose filtered by the kidney in a healthy individual is reabsorbed and returned to the blood circulation. Renal glucose reabsorption is predominantly mediated by SGLT2. Evidence suggests that in patients with T2DM, the expression and activity of SGLT2 is increased in the presence of hyperglycaemia, resulting in additional glucose reabsorption and preservation of elevated blood glucose levels. Pharmacologic inhibition of SGLT2 in the kidney reduces the capacity for renal glucose reabsorption by up to 50% [25-28].
As SGLT2 reabsorbs sodium and glucose in a co-transport manner, SGLT2-I also cause natriuresis and diuresis with concomitant blood pressure and body weight lowering effects [29]. The use of these specific SGLT2-I can decrease the glomerular filtration rate via restoration of the TGF. These treatments are associated with an initial decrease in glomerular filtration rate (GFR), followed by normalization and stabilization of the GFR [29,30]. Dapagliflozin was the first SGLT2-I approved in Europe in 2012 and therefore the one with the longest usage in clinical practice. Dapagliflozin, a potent and selective SGLT2-I, has been shown to have synergistic glycemic control in patients with type 2 diabetes when used in combination with metformin, or insulin [31-33].
SGLT2 inhibitors and glomerular hyperfiltration
Treatment of T2DM continues to present challenges, with a significant number of patients using different oral anti-diabetic drugs in combination with insulin, but without achieving the target HbA1c. Despite the availability of new generation insulin and oral anti-diabetic agents, therapeutic efficacy is also offset by side effects such as weight gain and recurrent hypoglycaemia episodes, and the number of patients reaching ESRD and replacement therapy are increasing every year.
The two most important pathophysiological mechanisms leading to renal hyperfiltration in diabetes are glomerular hemodynamic abnormalities due to neurohormonal activation and tubular factors. The hemodynamic hypothesis is based on changes in afferent and efferent arteriolar tone, resulting in glomerular hyperfiltration, mostly due to RAAS activation [34]. The tubular hypothesis is based on the fact that hyperglycaemia causes an increase in proximal tubule glucose filtered load in hyperglycaemic state. This results in over-activity of SGLT2 and consequent increased proximal tubular reabsorption of glucose and sodium and activation of the tubuloglomerular feedback system. This increased proximal sodium reabsorption leads to decreased sodium delivery to the macula densa, with consequent reduction in ATP breakdown and adenosine production. Adenosine is a strong vasoconstrictor, and its reduction as in hyperglycaemia causes vasodilation of the afferent arteriole and thus glomerular hyperfiltration.
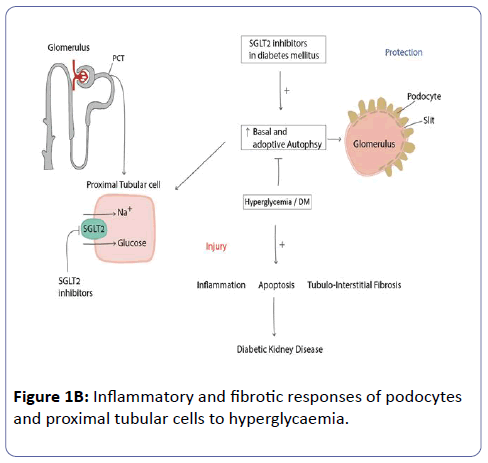
However, the renal protection could have been due to the known effects of Empagliflozin to reduce hyperfiltration and presumably intra-glomerular pressure with consequent renal tissue protection and slow kidney disease progression. The SGLT2 inhibitors, in addition to renal hemodynamic effects, including reducing glomerular hyperfiltration, also have inhibitory effects on the inflammatory and fibrotic responses of podocytes and proximal tubular cells to hyperglycaemia (Figure 1B) [35].
SGLT2 inhibitors and the diabetic kidney disease
The renal outcomes trial by Wanner et al. [36] recently reported that Empagliflozin (EMPA) administration was also associated with significant reductions in the progression of diabetic kidney disease including the rate of decline in eGFR, progression of albuminuria, and initiation of renal replacement therapy. There was a 38% risk reduction for progression to macroalbuminuria and a 44% relative risk reduction in doubling of serum creatinine in the EMPA group compared to placebo. Other studies demonstrate that in patients with eGFR of 30–60 mL/min/1.73 m2 the efficacy of SGLT2 inhibitors in reducing HbA1c is less than in patients with preserved GFR. Moreover, in patients with eGFR of 45 mL/min/1.73 m2, SGLT2 inhibitors are not recommended, and for this reason discontinuation is recommended when the eGFR is less than 45 m/Min.
The changes in GFR during SGLT2 inhibition are similar in patients with normal renal function and in those with mild to moderate CKD. In early stages of treatment, there is rapid decrease in GFR during the first weeks of treatment, followed by a progressive recovery that is faster and more evident in patients with normal renal function than with low GFR at baseline. Studies performed in patients with moderate renal impairment demonstrated significant renal protective effects on albuminuria and eGFR [37,38]. Overall, different studies consistently demonstrate a significant reduction in UACR during SGLT2 inhibition and stabilization of GFR. These renal effects occur early after treatment initiation and last, in the longest trial, for up to 2 years [39].
Studies in animal models with diabetic nephropathy
Hyperglycaemia-mediated alterations of intracellular metabolism, including the accumulation of advanced glycation end products (AGEs), activation of protein kinase C (PKC), and oxidative stress (free radical species) are the major contributing factors to the pathogenesis of DN. Chronic hyperglycaemia also activates the diacylglycerol–PKC pathway, which contributes to the regulation of vascular permeability, vasoconstriction, extracellular matrix (ECM) synthesis and turnover, cell growth, angiogenesis, and cytokine activation.
Other cellular insults from hyperglycaemia, apoptosis, autophagy, and cell cycle arrest, are supposed to play fundamental roles in initiation and progression of diabetic nephropathy. Apoptosis (programmed cell death) and autophagy (cellular process that transfers intracellular components to lysosomes for degradation to keep homeostasis and cell integrity) are the main pathways involved in DN. Autophagy, a well-coordinated multi-step process regulated by autophagyrelated gene (Atg) products (Atg5/Atg13), serves as an essential mechanism to maintain homeostasis of glomeruli and tubules, and plays important roles in human health and diseases such as high glucose and diabetes mellitus. Impairment of autophagy by up regulation of mTORC1 is implicated in the initiation and progression of DN.
During hyperglycaemia, increased glucose entry into renal proximal tubular cells stimulates oxidative stress generation with increased production of ROS and subsequently causes pro-apoptotic and pro-inflammatory processes. There is a decreased of number of podocytes and effusion due to decreased autophagy (podocytopathy). Therefore, insufficient autophagy against cellular stresses such as hyperglycaemia may lead to renal cells injuries. In addition, recent reports have shown that alterations in the pathways such as mTORC1, AMPK, and Sirt1 in renal cells are related to the pathogenesis of diabetic renal injuries. The activation of mTORC1 and the decreased activation of AMPK and Sirt1, in conditions such as hyperglycaemia, may result in an impairment of autophagy. Thus, adaptive autophagy may protect renal cells, including podocytes, mesangial cells, glomerular endothelial cells, and tubular epithelial cells, against cellular stresses under diabetic conditions; therefore, regulating autophagy may be a therapeutic option in diabetic nephropathy in the future.
In hyperglycaemias there is increased mTORC1 expression in podocytes and PCT cells with consequent decreased autophagic activity with renal injury and fibrosis. Thus, autophagy activation in tubular epithelial cells contributes to maintenance of cellular and organelle homeostasis/stress resistance, preventing tubulo-interstitial fibrosis, apoptosis, and inflammation in the podocytes as described with rapamune treatment in animals. SGLT2 inhibition may be expected to induce autophagy in proximal tubular epithelial cells and podocytes in diabetic kidney disease by down regulation of the mTORC1 pathway.
In the diabetic mouse model, the increased creatinine clearance in diabetic ob/ob mice was associated with significant glomerular hypertrophy (using PAS-stained kidney sections) compared with WT mice. Empagliflozin treatment of ob/ob mice led to a significant reduction of the glomerular and tubular basement membrane hypertrophy. Hyper-albuminuria in the hyperfiltration state was markedly decreased in Empagliflozintreated BTBR ob/ob mice. Glomerular matrix expansion in the diabetic BTBR ob/ob mice treated with Empagliflozin (SGL2 Inhibitor) showed a significant decrease in mesangial expansion compared with WT mice. Similar to the effects on albuminuria and glomerular hypertrophy, Empagliflozin treatment was able to ameliorate the increased glomerular matrix expansion. These findings suggest that SGLT2 inhibitors may protect against tubulointerstitial injury and fibrosis in diabetes if started in early stages of DM. Interestingly, there was a dose-dependent decrease in mesangial expansion and interstitial fibrosis, accompanied by reductions in macrophage infiltration, the gene expression of mediators of inflammation and oxidative stress, and transforming growth factor-β1 (TGFβ1). Taken together, results in animal models suggest that SGLT2 inhibition has hemodynamic effects, attenuating glomerular hyperfiltration and reducing albuminuria, and progression of DN. At the cellular level, activation of autophagy by the suppression of mTORC1 may be a novel therapeutic target for treatment of diabetic nephropathy, such as treatment with the mTORC1 inhibitor Rapamycin or SGLT2 inhibitors and Metformin. Rapamycin and metformin improved proteinuria and slow the progression of DN [40,41].
Therefore, improvement of autophagy may be a novel therapeutic option for the suppression of diabetic nephropathy through activation of mTORC1 and reduction of AMPK and Sirt1, which use impairment of autophagy in concomitant use of RAAS blockers. Therefore, an mTORC1 inhibitor, or AMPK and Sirt1 activators, may be important for slowing of diabetic nephropathy [42].
Conclusions
The prevalence of type 2 diabetes is expected to continue to increase significantly over the next few years. Microalbuminuria and serum NGAL with Cystatin C are in clinical use to predict early renal parenchymal damage, and to encourage early treatment. Abdel Razek and his group describe a novel method of diffusion tensor imaging to detect early renal parenchymal damage. There was a significant difference in the diffusion MR imaging of the renal cortex in diabetic patients compared to volunteers, and patients with macroalbuminuria compared to micro-normoalbuminuria. Also, they describe a good correlation of these imaging parameters in the diabetic kidney with urinary and serum biomarkers of diabetic patients.
The use of new anti-diabetic SGLT2 inhibitors creates a new era of diabetes mellitus treatment and reduction in micro- and macro-vascular complications. They are efficacious in monotherapy and function as well as add-ons to oral anti-hyperglycaemic agents. SGLT2 inhibitors can be effectively combined with insulin and Metformin for individuals of all ages and at all stages of type 2 diabetes. It is anticipated that SGLT2 inhibitors will be increasingly prescribed, given the associated promising reductions in cardiovascular and renal events due to strict control of glucose and the extra glucose effect on cellular defence mechanisms such as apoptosis and autophagy [43-46].
References
- Umanath K, Lewis JB (2018) Update on Diabetic Nephropathy: Core Curriculum. Am J Kidney Dis S0272-6386.
- Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic Kidney Disease Challenges, Progress, and Possibilities. Clin J Am Soc Nephrol 12: 2032–2045.
- Lin YC, Chang YH, Yang SY, Wu KD, Chu TS (2018) Update of pathophysiology and management of diabetic kidney disease. J Formos Med Assoc S0929-6646.
- Gilbert R (2013) Sodium-glucose linked transporter 2 inhibitors: potential for renoprotection beyond blood glucose lowering? Kidney Int 86: 693-700.
- Parving HH, Mauer M, Fioretto P, Rossing P, Ritz E (2012) Diabetic nephropathy. In: Brenner BM, (eds) Brenner & Rector’s the Kidney. 9h ed. Philadelphia, PA: Saunders Elsevier 1411–1154.
- Berezin A (2016) Metabolic memory phenomenon in diabetes mellitus: Achieving and perspectives. Diabetes Metab Syndr 10: S176-S183.
- Fioretto P, Mauer M (2007) Histopathology of diabetic nephropathy. Semin Nephrol 27: 195–207.
- Fioretto P, Mauer M, Brocco E, Velussi M, Frigato F, et al. (1996) Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia 39: 1569–1576.
- Doshi SM, Friedman AN (2017) Diagnosis and Management of Type 2 Diabetic Kidney Disease. Clin J Am Soc Nephrol 12: 1366-1373.
- Ng YP, Balasubramanian GP, Heng YP, Kalaiselvan M, Teh YW, et al. (2017) Renin angiotensin-aldosterone system (RAAS) blockers usage among type II diabetes mellitus patients-A Retrospective Study. Diabetes Metab Syndr 12: 305-308.
- Bacci MR, Chehter EZ, Azzalisb LA, de Aguiar Alves BC, and Fonseca FLA (2017) Serum NGAL and Cystatin C Comparison With Urinary Albumin-to-Creatinine Ratio and Inflammatory Biomarkers as Early Predictors of Renal Dysfunction in Patients With Type 2 Diabetes. Kidney Int Rep 2: 152–158.
- Razek AA, Al-Adlany M, Alhadidy A, Atwa M, Abdou N (2017) Diffusion tensor imaging of the renal cortex in diabetic patients: correlation with urinary and serum biomarkers. Abdom Radiol 42: 1493–1500.
- Maneiro LL, García PA (2015) Renin-Angiotensin-Aldosterone System Blockade in Diabetic Nephropathy. Present Evidences. J Clin Med 4: 1908-1937.
- Roscioni SS, Heerspink HJ, de Zeeuw D (2014) The effect of RAAS blockade on the progression of diabetic nephropathy. Nat Rev Nephrol 10: 77-87.
- Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R (2016) SGLT2 Inhibitors and the diabetic kidney. Diabetes Care 39: S165–S171.
- Toto RD (2017) SGLT-2 Inhibition: A Potential New Treatment for Diabetic Kidney Disease? Nephron 137: 64–67.
- Kawanami D, Matoba K, Takeda Y, Nagai Y, Akamine T, et al. (2018) SGLT2 Inhibitors as a Therapeutic Option for Diabetic Nephropathy. Int J Mol Sci 18.
- Fioretto P, Zambon A, Rossato M, Busetto L and Vettor R (2016) SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 39: S165-S171.
- Shen Z, Fang Y, Xing T, Wang F (2017) Diabetic nephropathy: From pathophysiology to treatment. J Diabetes Res 2379432.
- Salgado DMB, Guerra RAF (2014) Diabetic nephropathy and inflammation. World J Diabetes 5: 393-398.
- Elmarakby AA, Sullivan JC (2012) Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther 30: 49-59.
- Kitada M, Ogura Y, Monno I, Koya D (2017) Regulating Autophagy as a Therapeutic Target for Diabetic Nephropathy. Curr Diab Rep 17: 53.
- Hamzawy M, Gouda SAA, Rashid L, Morcos AM, Shoukry H, et al. (2017) The cellular selection between apoptosis and autophagy: roles of vitamin D, glucose and immune response in diabetic nephropathy. Endocrine 58: 66-80.
- Nistala R, Raja A, Pulakat L (2017) mTORC1 inhibitors rapamycin and metformin affect cardiovascular markers differentially in ZDF rats. Can J Physiol Pharmacol 95: 281-287.
- Wanner C (2017) EMPA-REG OUTCOME: The nephrologist’s point of view. Am J Cardiol 120: S59-S67.
- Bailey C, Iqbal N, T’joen C, List J (2012) Dapagliflozin monotherapy in drug naÃÆââ¬âÃâà ¸ve patients with diabetes: a randomized-controlled trial of low-dose range. Diabetes Obes Metab 14: 951–959.
- Cherney D, Perkins B, Soleymanlou N, Maione M, Lai V, et al. (2014) Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597.
- Miller EM (2017) Elements for success in managing Type 2 Diabetes with SGLT-2 inhibitors: Role of the kidney in glucose homeostasis: Implications for SGLT-2 inhibition in the treatment of Type 2 Diabetes Mellitus. J Fam Pract 66: S3-S5.
- Peene B, Benhalima K (2014) Sodium glucose transporter protein 2 inhibitors: focusing on the kidney to treat type 2 diabetes. Ther Adv Endocrinol Metab 5: 124–136.
- Baker W, Smyth L, Riche D, Bourret E, Chamberlin K, White W (2014) Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 8: 262–275.
- ŠkrtiÃÆââ¬Å¾Ãâââ¬Â¡ M, Cherney DZ (2015) Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens 24: 96–103.
- Bailey C, Gross J, Pieters A, Bastien A, List J (2010) Effect of dapagliflozin in patients with type 2 diabetes who have inadequate control with metformin a randomised, double-blind, placebo-controlled trial. Lancet 375: 2223–2233.
- Barnett A, Mithal A, Manassie J, Jones R, Rattunde H, et al. (2014) Efficacy and safety of empagliflozin when added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomized, doubleblind, placebo controlled trial. Lancet Diabetes Endocrinol 2: 369–384.
- Garg SK, Henry RR, Banks P, Buse JB, Davies MJ, et al. (2017) Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N Engl J Med 377: 2337-2348.
- Thomson SC, Blantz RC (2008) Glomerulotubular balance, tubuloglomerular feedback, and salt homeostasis. J Am Soc Nephrol 19: 2272–2275.
- Heerspink HJ, Desai M, Jardine M, Balis D, Meininger G, et al. (2017) Canagliflozin Slows Progression of Renal Function Decline Independently of Glycemic Effects. J Am Soc Nephrol 28: 368-375.
- Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, et al. (2016) Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 375: 323-334.
- Jaikumkaoa K, Pongchaidechaa A, Chatsudthipong V, Chattipakorn SC, Chattipakorn N, et al. (2017) The roles of sodium-glucose cotransporter 2 inhibitors in preventing kidney injury in diabetes. Biomed Harmacother 94: 176–187.
- Komala MG, Panchapakesan U, Pollock C, Mather A (2013) Sodium glucose cotransporter 2 and the diabetic kidney. Curr Opin Nephrol Hypertens 22: 113–119.
- De Nicola L, Gabbai F, Liberti M, Sagliocca A, Conte G, et al. (2014) Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes. Am J Kidney Dis 64: 16–24.
- Gembardt F, Bartaun C, Jarzebska N, Mayoux E, Todorov VT, et al. (2014) The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol 307: F317–F325.
- Ishibashi Y, Matsui T, Yamagishi SI (2016) Tofogliflozin, a selective inhibitor of sodium-glucose cotransporter 2, suppresses renal damage in KKAy/Ta mice, obese and type 2 diabetic animals. Diab Vasc Dis Res 13: 438-441.
- Ferrannini E, Berk A, Hantel S, Pinnetti S, Hach T, et al. (2013) Long-term safety and efficacy of empagliflozin, sitagliptin and metformin: an active-controlled, parallel group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care 36: 4015–4021.
- Woo VC, Berard LD, Bajaj HS, Ekoé JM, Senior PA (2018) Considerations for Initiating a Sodium-Glucose Co-Transporter 2 Inhibitor in Adults With Type 2 Diabetes Using Insulin.Can J Diabetes 42: 88-93.
- Davidson JA, Sloan L (2017) Fixed-Dose Combination of Canagliflozin and Metformin for the Treatment of Type 2 Diabetes: An Overview. Adv Ther 34: 41-59.
- Kitada M, Ogura Y, Monno I, Koya D (2017) Regulating autophagy as a therapeutic target for diabetic nephropathy. Curr Diab Rep 17: 53.
- Hocher B, Tsuprykov O (2017) Diabetic nephropathy: Renoprotective effects of GLP1R agonists and SGLT2 inhibitors. Nat Rev Nephrol 13: 728-730.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences