Nephropreventive Effect of Shikonin on Murine LPS-induced Septic Acute Kidney Injury via Nrf2 Activation with Antioxidative Responses
Madoka Kawara, Rika Matsunaga, Yuko Yamamoto, Go Yoneda, Rika Fujino,Kazuhiko Nishi,Hirofumi Jono and Hideyuki Saito
DOI10.21767/2472-5056.100019
Madoka Kawara1, Rika Matsunaga1, Yuko Yamamoto1, Go Yoneda1, Rika Fujino2, Kazuhiko Nishi3, Hirofumi Jono1,2 and Hideyuki Saito1,2*
1Department of Clinical Pharmaceutical Sciences, Graduate School of Pharmaceutical Sciences, Kumamoto University, Kumamoto, Japan
2Department of Pharmacy, Kumamoto University Hospital, Kumamoto, Japan
3Department of Hemo-Dialysis, Kumamoto University Hospital, Kumamoto, Japan
- *Corresponding Author:
- Hideyuki Saito
PhD, Department of Pharmacy, Kumamoto University Hospital
1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan
Tel: +81-96-373-5820
E-mail: saitohide@fc.kuh.kumamoto-u.ac.jp
Received date: September 05, 2016; Accepted date: September 21, 2016; Published date: September 26, 2016
Citation: Kawara M, Matsunaga R, Yamamoto Y, Yoneda G, Fujino R, et al. (2016) Nephropreventive Effect of Shikonin on Murine LPS-induced Septic Acute Kidney Injury via Nrf2 Activation with Antioxidative Responses. J Clin Exp Nephrol 1:19. DOI: 10.21767/2472-5056.100019
Copyright: © 2016 Kawara M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The present study explored the nephropreventive effect of shikonin, a naturally occurring herbal medicine, possessing proteasome inhibitory and antioxidant effects via activation of nuclear factorerythroid 2-related factor 2 (Nrf2) against lipopolysaccharide (LPS)-induced septic acute kidney injury (AKI) using a murine model.
Methods: Septic AKI was induced in C57BL mice by intraperitoneal administration of LPS (20 mg/kg). Shikonin (5 mg/kg) was administered intraperitoneally to mice 1 hr before the LPS treatment. Development of renal injury, Nrf2 activation and antioxidative responses (heme oxygenase-1; HO-1 and NAD(P)H: quinone oxidoreductase; NQO1) in the kidney of LPS-treated mice with or without shikonin were compared.
Results: Serum levels of Interleukin (IL)-6 and tissue necrotic factor (TNF)-α were markedly elevated in LPStreated mice. However, shikonin administration resulted in a significant decrease in the normally elevated levels of these cytokines. Survival rates of LPS-treated mice and LPS- and shikonin-treated mice were 36 and 82%, respectively. Serum creatinine and blood urea nitrogen (BUN) markedly increased in LPS-treated mice, whereas shikonin improved these renal function markers. Histochemical examination revealed that glomerular and tubular injuries of LPS-treated mice were reduced by shikonin. Serum hydroperoxide and renal malondialdehyde levels were markedly increased by LPS treatment, whereas shikonin significantly suppressed these oxidative stress markers. Shikonin administration induced a marked expression of Nrf2 in the renal nuclear fraction, which was associated with significant increases in mRNA expression of HO-1 and NQO1.
Conclusion: These results suggest that shikonin could be a potential nephropreventive agent against septic AKI, at least in part, through the transient activation of renal Nrf2 followed by induction of its downstream antioxidant molecules.
https://marmaris.tours
https://getmarmaristour.com
https://dailytourmarmaris.com
https://marmaristourguide.com
https://marmaris.live
https://marmaris.world
https://marmaris.yachts
Keywords
Sepsis; Lipopolysaccharide; Acute kidney injury; Nrf2; Oxidative stress; Shikonin
Introduction
Lipopolysaccharide (LPS) is an endotoxin produced after infection by Gram negative bacteria, which can cause sepsis. The presence of LPS in the body stimulates the production of various cytokines and reactive oxygen species. As a result, there is a cascade of events including overproduction of inducible nitric oxide synthase (iNOS), low blood pressure with systemic vasodilatation, decreased endothelial wall-dependent NO synthesis, renal tubular injury and damage to the glomerulus, resulting in severe acute kidney injury (AKI) [1-3]. Sepsis-induced AKI often develops in patients with decreased immunocompetence, resulting in severe renal failure and exacerbated mortality. Septic AKI is treated by infusion therapy and/or anti-inflammatory treatments. However, the efficacy of these treatments is insufficient to improve survival rates [1-3]. Acute renal failure is associated with elevated serum levels of malondialdehyde (MDA), an oxidative stress marker, whereas the serum level of selenium, an antioxidant metal, is significantly lowered [4]. In experiments using an animal model, it was reported that expression of superoxide dismutase (SOD), an antioxidant enzyme, decreases as a result of kidney dysfunction [5]. It has been suggested that the increase in oxidative stress can mediate the onset of sepsisinduced AKI associated with an elevation of inflammatory cytokines [1]. Therefore, in order to improve mortality rates in sepsis-induced AKI, it is important to consider treatment with a therapeutic drug having an antioxidant effect in addition to an anti-inflammatory effect.
Shikonin, a naturally occurring herbal medicine extracted from the red-root gromwell, displays potent proteasome inhibitory and antioxidant effects [6,7]. It has been proposed that shikonin may exert its anti-inflammatory potency in LPSmediated acute lung injury by inhibiting the NF-κB signaling pathway, which mediates the expression of pro-inflammatory cytokines [8]. In addition, it was reported that the neuroprotective effects of shikonin against cerebral ischemia/ reperfusion injury could be attributed to its antioxidant effect [9]. By contrast, shikonin has been shown to induce apoptotic death of some cancer cell lines in culture through increasing the intracellular levels of reactive oxygen species (ROS) [10,11]. ROS production by shikonin resulted in the inhibition of nuclear translocation or activation of nuclear factorerythroid 2-related factor 2 (Nrf2) [10]. Thus, the pharmacotoxicological properties of shikonin and its molecular effect on Nrf2 expression have not yet been fully elucidated.
In the present study, the pharmacological effect of shikonin on development of septic AKI in an LPS-treated murine model was examined from the viewpoint of its anti-inflammatory and oxidative potencies.
Materials and Methods
Shikonin was obtained from Enzo Life Sciences, Inc. (Farmingdale, NY). Lipopolysaccharide (LPS) was purchased from Sigma Aldrich, Co. (St. Louis, USA). All other chemicals used in this study were of analytical grade.
All methods and procedures for animal studies were approved by Kumamoto University Ethical Committee concerning animal experiments. Animals were treated in conformity to the Guidelines of the United States National Institutes of Health regarding the care and use of experimental animals, and the Guidelines of Kumamoto University for the care and use of laboratory animals. C57BL/6J male mice at 7 weeks of age were kept in a standard animal maintenance facility at a constant temperature (22 ± 2°C), humidity (50-70%) and a 12/12 hr light/dark cycle for about a week before the day of the experiment, with food and water available ad libitum. Mice were divided into three different groups as follows: sham-operated mice (control mice), mice treated with LPS (20 mg/kg, intraperitoneal (i.p.) injection), and shikonin (5 mg/kg, i.p. injection)-administered mice treated with LPS (20 mg/kg, i.p. injection). Shikonin was administered to mice 1 hr before LPS treatment, and blood was collected 4 hr, 24 hr and 48 hr after LPS treatment. Kidney was harvested 48 hr after LPS treatment. All administrations, sacrifices and sample collections were performed under surgical anesthesia using diethyl ether. Blood was collected from the abdominal aorta and centrifuged at 3,000g for 10 min to obtain a serum sample. Levels of serum creatinine (SCr) (enzymatic method) and blood urea nitrogen (BUN) (uricase ultraviolet (UV) method) were determined. Kidneys were harvested 48 hr after LPS treatment and homogenized in phosphate-buffered saline (PBS, pH 7.4) using a Polytron PT3000 homogenizer (Kinematica AG, Lucerne, Switzerland). Serum levels of interleukin-6 (IL-6) and tissue necrotic factor (TNF)-α were determined using Mouse IL-6 Quantikine ELISA Kit and Mouse TNF-α Quantikine ELISA Lit (R&D Systems, Inc., Minneapolis, MN), respectively.
Paraffin-embedded 4 μm-thick sections of kidney were used for hematoxylin-eosin (HE) staining and immunoblot analysis. Immunoblot analysis for Nrf2 was performed with extracted nuclear fractions of kidneys using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Rockford, IL). The nuclear fractions were combined with loading buffer (2% w/v SDS, 125 mM Tris-HCl, 20% v/v glycerol and 5% v/v 2 mercaptoethanol) and heated at 95°C for 2 min. Samples were subjected to SDS-PAGE and transferred onto a PVDF membrane. The blots were incubated with a primary antibody specific for Nrf2 (Santa Cruz Biotechnology, Inc., TX) and then washed with Tris-buffered saline containing 0.3 v/v% Tween 20 before incubation with the secondary antibody (horseradish peroxidase-linked anti-rabbit immunoglobulin F(ab)2 or horseradish peroxidase-linked anti-mouse immunoglobulin F(ab)2 (GE Healthcare) for 1 hr at room temperature. Immunoblots were visualized with an ECL system (ECL Advance Western Blotting Detection Kit; GE Healthcare, Little Chalfont, UK).
The extraction of mRNA from kidney tissue samples was performed by a phenol-chloroform extraction method using Trizol (Thermo Fisher Scientific). After adding Trizol, kidney sample was homogenized in FastPrep (Funakoshi, Co., Ltd., Tokyo) and centrifuged (12,000 rpm, 3 min) to obtain a supernatant. 200 μl of chloroform was added to the supernatant and the mixture was vortexed. After incubation at room temperature for 10 min the mixture was centrifuged at 4°C (12,000 rpm, 12 min) to obtain a supernatant. Upon addition of 250 μl of isopropanol to the aqueous layer, the supernatant was incubated at room temperature for 5 minutes. After centrifugation at 4°C (12,000 rpm for 10 min) the supernatant was discarded and 500 μl of 70% ethanol was added to the precipitate. The sample was then centrifuged again at 4°C (7,500 rpm for 5 min) before removing the ethanol and allowing the pellet to air-dry. The obtained pellet was dissolved in RNase-free water. cDNA was prepared from the purified mRNA by reverse-transcription using PrimeScript reagent kit (TAKARA BIO Inc., Osaka, Japan). Real-time PCR reaction was performed using cDNA in 100 μL of mixture containing 5 μL SYBR® premix DimerEraserTM, 0.3 μL of PCR forward primer (10 μM), 0.3 μL PCR reverse primer (10 μM), 1 μL of cDNA and 3.4 μL of distilled water under the following conditions: 95°C for 10 min, followed by 50 cycles of 95°C for 5 s, 72°C for 30 s and 55°C for 30 s. The primers were purchased from Nihon Gene Research Laboratories Inc. (Miyagi, Japan). The primer sequences for each gene were as follows: heme oxygenase (HO)-1, forward 5’-AACAAGCAGAACCCAGTCTATGC-3’ and reverse 5’- AGGTAGCGGGTATATGCGTGGGCC-3’; NAD(P)H:quinone oxidoreductase (NQO)-1, forward 5’- AGGGTTCGGTATTACGATCC-3’ and reverse 5’- AGTACAATCAGGGCTCTTCTCG-3’. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and 18S ribosomal RNA were used as internal references for normalization of relative mRNA amounts.
Renal accumulation of MDA, a marker of lipid peroxidation, was determined as thiobarbituric acid-reactive substances (TBARS). Kidney tissues were homogenized in ice-cold lysis buffer containing 50 mM Tris pH 7.4, 150 mM EDTA, 1% Triton X-100, 1% sodium deoxy cholate and 0.1% SDS. TBARS content was determined according to the manufacturer’s instructions (TBARS Assay Kit; Cayman Chemical, Ann Arbor, MI).
Serum hydroperoxide concentration was determined as reactive oxygen molecules using a Free Radical Analytical System according to the manufacturer’s instructions (Wismerll Co. Ltd., Tokyo, Japan).
Statistical analysis
Data were analyzed statistically by analysis of variance, followed by Scheffé’s multiple comparison tests. A P value of < 0.05 was considered statistically significant. All data were represented as the mean ± SD.
Results
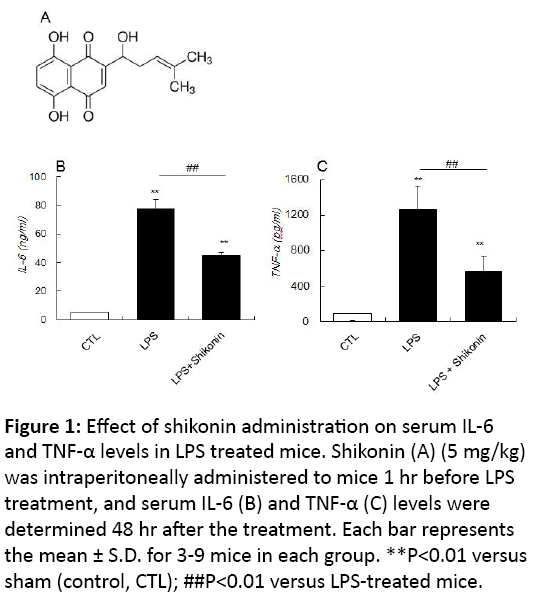
The influence of LPS treatment with or without shikonin administration on serum IL-6 and TNF-α levels 4 hr after treatment was examined. In the LPS-treated mice, serum IL-6 levels significantly increased, although this increase was much reduced in shikonin administered mice. In addition, serum TNF-α levels significantly increased in LPS-treated mice but this was substantially reduced in shikonin-administered mice (Figure 1).
Figure 1: Effect of shikonin administration on serum IL-6 and TNF-α levels in LPS treated mice. Shikonin (A) (5 mg/kg) was intraperitoneally administered to mice 1 hr before LPS treatment, and serum IL-6 (B) and TNF-α (C) levels were determined 48 hr after the treatment. Each bar represents the mean ± S.D. for 3-9 mice in each group. **P<0.01 versus sham (control, CTL); ##P<0.01 versus LPS-treated mice.
These results suggested that shikonin indeed exhibited its anti-inflammatory effect by suppressing the LPS-induced generation of inflammatory cytokines, IL-6 and TNF-α.
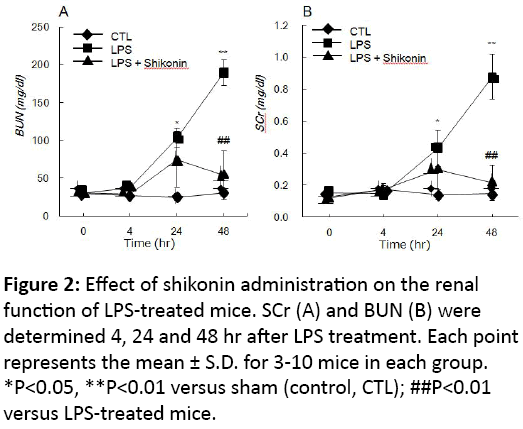
The survival rate of mice after LPS treatment was 35.7% (5/14 mice), but increased to 81.8% (9/11 mice) in the LPS treatment with shikonin administration group. This observation suggested that shikonin improves the survival rate of mice treated with LPS. After LPS treatment, BUN and SCr were significantly elevated at 24 hr and 48 hr in a timedependent manner (Figure 2). By contrast, although LPStreated mice with shikonin administration also showed an initial increase in both BUN and SCr compared to those of the control (sham) mice, the level of these markers were significantly reduced to that seen in the control within 48 hr (Figure 2).
Figure 2: Effect of shikonin administration on the renal function of LPS-treated mice. SCr (A) and BUN (B) were determined 4, 24 and 48 hr after LPS treatment. Each point represents the mean ± S.D. for 3-10 mice in each group. *P<0.05, **P<0.01 versus sham (control, CTL); ##P<0.01 versus LPS-treated mice.
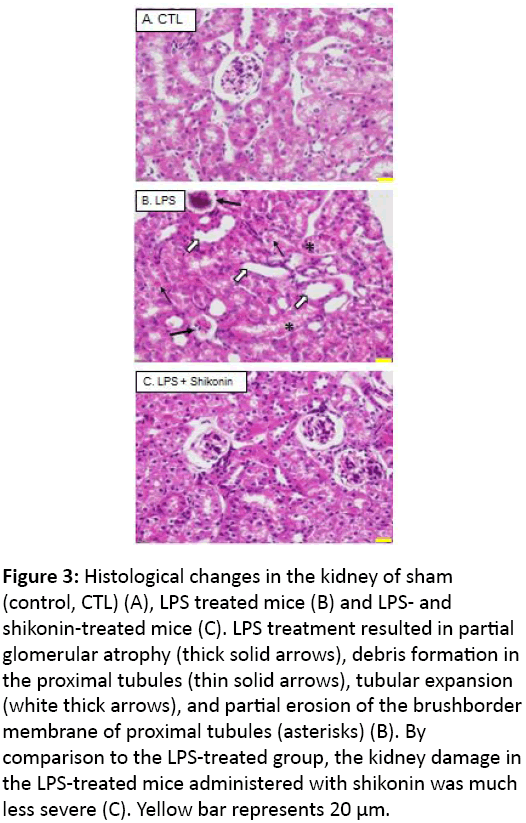
Histological analyses revealed partial glomerular atrophy and debris formation in tubules, renal tubular expansion and partial erosion of the apical membrane of the renal tubules in LPS treated mice. However, these injuries were partly improved in the mice with shikonin administration, suggesting that shikonin displays a nephroprotective effect on the development of LPS-induced AKI (Figure 3).
Figure 3: Histological changes in the kidney of sham (control, CTL) (A), LPS treated mice (B) and LPS- and shikonin-treated mice (C). LPS treatment resulted in partial glomerular atrophy (thick solid arrows), debris formation in the proximal tubules (thin solid arrows), tubular expansion (white thick arrows), and partial erosion of the brushborder membrane of proximal tubules (asterisks) (B). By comparison to the LPS-treated group, the kidney damage in the LPS-treated mice administered with shikonin was much less severe (C). Yellow bar represents 20 μm.
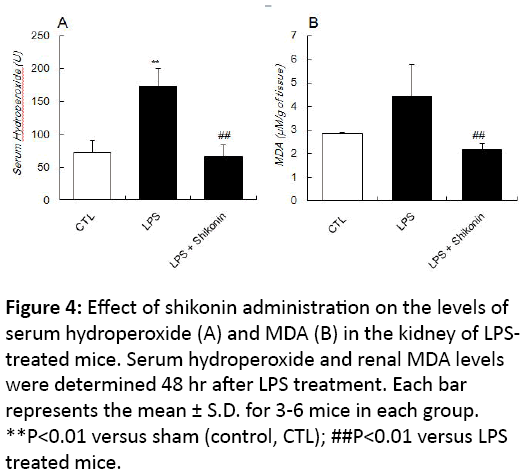
In addition to inflammatory response, endotoxins produced after bacterial infection leads to the generation of ROS in association with various oxidation stress markers. To elucidate whether shikonin also shows antioxidative effect on LPSinduced kidney injury, we next measured the level of a lipid peroxidation marker, MDA, in renal tissue as well as the serum hydroperoxide level in LPS-treated mice with or without shikonin administration. LPS-treated mice showed a marked increase in the level of serum hydroperoxide, and the MDA level in the kidney also tended to increase (Figure 4).
Figure 4: Effect of shikonin administration on the levels of serum hydroperoxide (A) and MDA (B) in the kidney of LPStreated mice. Serum hydroperoxide and renal MDA levels were determined 48 hr after LPS treatment. Each bar represents the mean ± S.D. for 3-6 mice in each group. **P<0.01 versus sham (control, CTL); ##P<0.01 versus LPS treated mice.
Discussion
We investigated the molecular and cellular mechanisms of the nephropreventive effect of shikonin using an LPS-treated sepsis-induced AKI mouse model. Based on the experimental method described in previous reports [12,13], shikonin was administered intraperitoneally 1 hr before LPS treatment. Serum and renal tissue were then collected or harvested for chemical and pathological analyses 4 hr, 24 hr and 48 hr after LPS treatment with or without shikonin administration. Our results suggested, in addition to its anti-inflammatory effect, shikonin may also exhibit oxidative stress suppressive effects through activation of the antioxidant defensive transcription factor Nrf2 in renal tissue. These findings help explain the nephropreventive effect of shikonin.
By contrast, mice given shikonin displayed significantly lower levels of serum hydroperoxide and renal tissue MDA in comparison with LPS-treated mice. These findings suggested that shikonin administration suppressed the oxidative stress evoked by LPS treatment in serum and renal tissue.
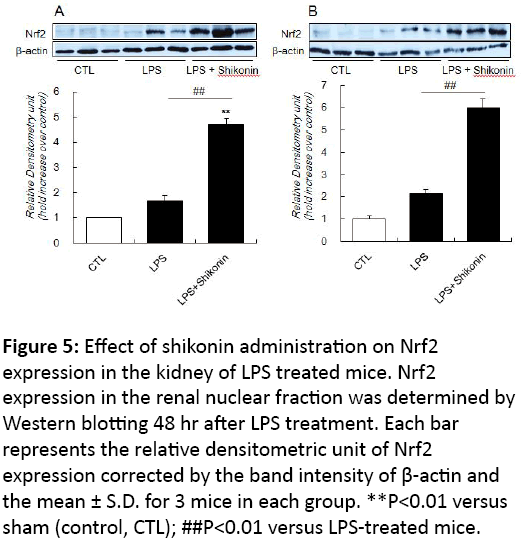
The activity of Nrf2 is primarily regulated through its interaction with keap1 in the cytoplasm, which acts as a repressor in the normal oxidative stress-free environment and is degraded by the proteasome. However, when tissues are subjected to stress by oxidation and/or inflammatory reaction, Nrf2 dissociates from keap1 and shifts into the nucleus, thereby promoting the expression of antioxidant proteins and detoxification enzyme genes through an antioxidative response element (ARE) and an electrophile responsive element (EpRE). Thus, we examined the influence of LPS treatment and shikonin administration on protein expression/ activation of Nrf2 in kidney. LPS treatment caused a slight, but not significant, increase in Nrf2 expression in both the cytosolic and nucleus fractions of the kidney (Figure 5). In contrast, Nrf2 expression was markedly elevated in the kidney of LPS-treated mice with shikonin administration compared with those of LPS-treated mice (Figure 5).
Figure 5: Effect of shikonin administration on Nrf2 expression in the kidney of LPS treated mice. Nrf2 expression in the renal nuclear fraction was determined by Western blotting 48 hr after LPS treatment. Each bar represents the relative densitometric unit of Nrf2 expression corrected by the band intensity of β-actin and the mean ± S.D. for 3 mice in each group. **P<0.01 versus sham (control, CTL); ##P<0.01 versus LPS-treated mice.
These findings suggest that shikonin stimulates not only cytosolic Nrf2 protein expression but its activation through a transient shift into the nucleus.
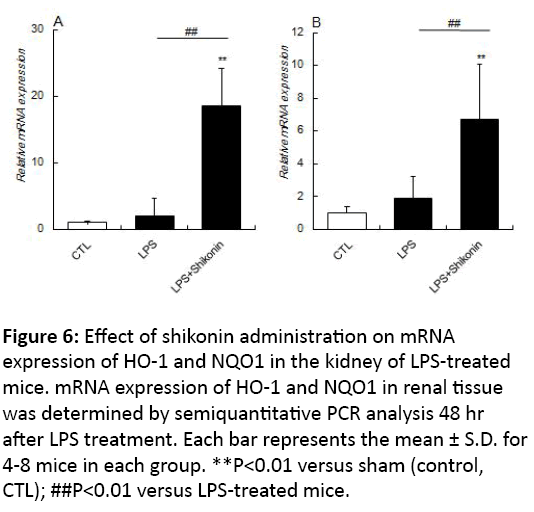
The presence of Nrf2 in the nucleus is known to upregulate the expression of various antioxidant proteins and detoxification enzymes, including HO-1 and NQO1 located downstream of the ARE/EpRE region. The kidney of LPStreated mice given shikonin showed a significant upregulation of both HO-1 and NQO-1 mRNA compared with those of control mice (sham) and mice treated with LPS alone (Figure 6). These findings are consistent with shikonin induced activation / expression of Nrf2.
Figure 6: Effect of shikonin administration on mRNA expression of HO-1 and NQO1 in the kidney of LPS-treated mice. mRNA expression of HO-1 and NQO1 in renal tissue was determined by semiquantitative PCR analysis 48 hr after LPS treatment. Each bar represents the mean ± S.D. for 4-8 mice in each group. **P<0.01 versus sham (control, CTL); ##P<0.01 versus LPS-treated mice.
Sepsis is an infection that has evoked systemic inflammatory response syndrome, which is caused by endogenous inflammatory mediators being released into the blood. Specifically, sepsis induces production of inflammatory cytokines, including IL-6 and TNF-α, by stimulation from a number of factors including the bacterial toxin LPS [14]. In the present study, levels of serum IL-6 and TNF-α were significantly elevated in sepsis-induced AKI model mice 4 hr after LPS treatment, indicating that the inflammatory response had been evoked (Figure 1). However, administration of shikonin to LPS-treated mice significantly decreased the levels of IL-6 and TNF-α, suggesting this compound displays a systemic antiinflammatory effect. Renal function levels were normal at 4 hr in the early onset stage of inflammation evoked by septic response (Figure 2). However, analysis of renal function markers at 24 hr and 48 hr after LPS treatment showed BUN and SCr levels had significantly increased (Figure 2). Nonetheless, this increase in BUN and SCr at 48 hr was markedly suppressed by administration of shikonin. Evaluation of renal function in the LPS group and LPS with shikonin group showed that the kidney injury caused by LPS treatment was significantly reduced by administration of shikonin. Moreover, shikonin also markedly improved the survival rate. Taken together these results suggested that shikonin displays a renal preventive effect in sepsis-related AKI induced by LPS treatment.
Next, we examined the likely mechanism for the nephropreventive effect of shikonin. ROS are generated during oxygen metabolism and are important in terms of the systemic defensive and supportive processes. However, excessive production of ROS is a pathological condition of oxidative stress, which can bring about the onset of various disease states. Therefore, antioxidative functions normally act to remove ROS generated in the body. Under normal conditions, ROS is removed immediately by an antioxidant mechanism. However, when excess ROS builds up in vivo, functional disorder of organs occurs by modification of nucleic acids, proteins and lipids, which changes the structure and function of various tissues including kidney [15]. Recent studies examining the participation of ROS in AKI reported that the cytotoxic agent peroxynitrite (ONOO-) increases when ROS or MDA reacts with NO in renal tissues [16]. Furthermore, symptoms of AKI have been reported to improve upon administration of agents that eliminate ROS, suggesting that ROS plays an important pathological role in the development and progress of AKI [17,18]. In the present study, we measured MDA in the kidney of sepsis-induced AKI mice as well as the serum levels of hydroperoxide. We found that the level of serum hydroperoxide increased significantly in LPS-treated mice, and MDA showed a slight elevation in renal tissue (Figure 4). However, shikonin administration significantly reduced the levels of both serum hydroperoxide and MDA in the kidney (Figure 4). These results suggested that ROS participates in the development of sepsis-induced AKI and that shikonin had an antioxidative action in the circulatory system and kidney.
Nrf2 is known as a master transcription factor that regulates the expression of various enzymes and transporters with an antioxidative function to remove ROS and related compounds in tissues, including kidney [19,20]. It is reported that Nrf2 contributes to cellular protection in septic model mice [21], and that Nrf2-knockout mice are more sensitive to LPS-induced inflammation [22]. Furthermore, activation of Nrf2 was found to suppress inflammatory cytokine-producing reactions, thereby leading to cytoprotective effects [23]. In the present study, we evaluated the level of Nrf2 in the cytoplasm and nucleus fractions of kidney 48 hr after LPS treatment (Figure 5). Our findings confirmed that expression of Nrf2 was increased in kidney of the LPS-treated group, and was significantly enhanced by shikonin administration. These observations suggested that the antioxidative mechanism involves Nrf2, which counteracts the excessive buildup of ROS caused by LPS-induced sepsis. Furthermore, we investigated the possibility that the protective effect on the kidney was provided by the antioxidant action of shikonin acting synergistically, which contributed to Nrf2 action. Nrf2 shifts into the nucleus when activated by ROS and upregulates expression of downstream genes HO-1 and NQO1 with an antioxidant function by interacting with the antioxidant responsive element (ARE) and electrophile responsive element (EpRE) [24]. In addition, it is reported that LPS-treated animals show upregulation of HO-1 expression, which helps protect against organ dysfunction [25]. Examination of the changes of HO-1 and NQO1 gene expression in the kidney 48 hr after LPS treatment showed both genes were upregulated in shikonin administered mice, but not in the LPS-treated group. Therefore, shikonin appears to stimulate the expression of HO-1 and NQO1 by directly activating Nrf2, resulting in a potential nephropreventive effect against AKI evoked by LPSinduced oxidative stress.
In conclusion, in addition to endogenous antioxidative mechanisms, shikonin could have an anti-inflammatory effect and antioxidative action via Nrf2 activation, leading to a nephropreventive effect against LPS-induced septic AKI. These findings could be useful in elucidating the molecular mechanism for the development and progress of sepsisinduced AKI. Furthermore, this study provides useful basic information for establishing a new therapeutic strategy to prevent sepsis-induced AKI.
Funding
This study was supported in part by grants from Japan Society for the Promotion of Science (JSPS) KAKENHI (JP25293040 and JP25670080 to H.S.); Japan Science and Technology Agency (JST) Accelerating Utilization of University IP Program (FY2013-300-148007 to H.S.).
References
- Heemskerk S, Masereeuw R, Russel FG, Pickkers P (2009) Selective iNOS inhibition for the treatment of sepsis-induced acute kidney injury. Nat Rev Nephrol 5: 629-640.
- Alobaidi R, Basu RK, Goldstein SL, Bagshaw SM (2015) Sepsis-associated acute kidney injury.SeminNephrol 35: 2-11.
- Zarbock A, Gomez H, Kellum JA (2014) Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies.CurrOpinCrit Care 20: 588-595.
- Metnitz GH, Fischer M, Bartens C, Steltzer H, Lang T (2000) Impact of acute renal failure on antioxidant status in multiple organ failure. ActaAnaesthesiolScand 44: 236-240.
- Jao HC, Lin YT, Tsai LY, Wang CC, Liu HW (2005) Early expression of heme oxygenase-1 in leukocytes correlates negatively with oxidative stress and predicts hepatic and renal dysfunction at late stage of sepsis. Shock 23: 464-469.
- Takano-Ohmuro H, Yoshida LS, Yuda Y, Morioka K, Kitani S (2008) Shikonin inhibits IgE-mediated histamine release by human basophils and Syk kinase activity. Inflamm Res 57: 484-488.
- Li Lu (2011) Shikonin extracted from medical Chinse herbs exerts anti-inflammatory effect via proteasome inhibition. Eur J Pharmacol 658: 242-247.
- Liang D, Sun Y, Shen Y, Li F, Song X, et al. (2013) Shikonin exerts anti-inflammatoy effects in a murine model of lipopolysaccharide-induced acute lung injury by inhibiting the nuclear factor-ÃÆïÃâÃÂÃâëB signaling pathway. IntImmunopharmacol 16: 475-480.
- Wang Z, Liu T, Gan L, Wang T, Yuan X, et al. (2010) Shikonin protects mouse brain against cerebral ischemia/reperfusion injury through its antioxidant activity. Eur J Pharmacol 643: 211-217.
- Yang JT, Li ZL, Wu JY, Lu FJ, Chen CH (2014) An oxidative stress mechanism of shikonin in human glioma cells.PLoS One 9: e94180.
- Ko H, Kim SJ, Shim SH, Chang H, Ha CH (2016) Shikonin Induces the Apoptotic Cell Death via Regulation of p53 and Nrf2 in AGS Human Stomach Carcinoma Cells.BiomolTher (Seoul) doi: 10.4062/biomolther.2016.008.
- Innamorato NG, Rojo AI, Garcia-Yague AJ, Yamamoto M, de Ceballos ML, et al. (2008) The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol 181: 680–688.
- Liang D, Sun Y, Shen Y, Li F, Song X, et al. (2013) Shikonin exerts anti-inflammatory effects in a murine model of lipopolysaccharide-induced acute lung injury by inhibiting the nuclear factor-ÃÆïÃâÃÂÃâëB signaling pathway. IntImmunopharmacol 16: 475-480.
- Wahab F, Tazinafo LF, Cárnio EC, Aguila FA, Batalhão ME, et al. (2015) Interleukin-1 receptor antagonist decreases cerebrospinal fluid nitric oxide levels and increases vasopressin secretion in the late phase of sepsis in rats. Endocrine 49: 215-221.
- Walker PD, Shah SV (1992) Evidence suggesting a role for hydroxyl radical in gentamicin-induced acute renal failure in rats. J Clin Invest 81: 334-341.
- Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, et al. (2001) Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol 281: F948-957.
- Nakajima T, Hishida A, Kato A (1994) Mechanisms for protective effects of free radical scavengers on gentamicin-mediated nephropathy in rats. Am J Physiol 266: F425-431.
- Yin M, Wheeler MD, Connor HD, Zhong Z, Bunzendahl H, et al. (2001) Dikalova A: Cu/Zn-superoxide dismutase gene attenuates ischemia-reperfusion injury in the rat kidney. J Am SocNephrol 12: 2691-2700.
- Joshi G, Johnson JA (2012) The Nrf2-ARE pathway: a valuable therapeutic target for the treatment of neurodegenerative diseases. Recent Pat CNS Drug Discov 7: 218-229.
- Saito H (2013) Toxico-pharmacological perspective of the Nrf2-Keap1 defense system against oxidative stress in kidney diseases. BiochemPharmacol 85: 865-872.
- Ha YM, Ham SA, Kim YM, Lee YS, Kim HJ, et al. (2011) β1-adrenergic receptor-mediated HO-1 induction, via PI3K and p38 MAPK, by isoproterenol in RAW 264.7 cells leads to inhibition of HMGB1 release in LPS-activated RAW 264.7 cells and increases in survival rate of CLP-induced septic mice. BiochemPharmacol 82: 769-777.
- Innamorato NG, Rojo AI, Garcia-Yague AJ, Yamamoto M, de Ceballos ML, et al. (2008) Cuadrado A: The transcription factor Nrf2 is a therapeutic target against brain inflammation. J Immunol 181: 680-689.
- Lin W, Wu RT, Wu T, Khor TO, Wang H, et al. (2008) Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. BiochemPharmacol 76: 967-973.
- Lee IS, Lim J, Gal J, Kang JC, Kim HJ, et al. (2011) Kang BY, Choi HJ: Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. NeurochemInt 58: 153-160.
- Lee CJ, Lee SS, Chen SC, Ho FM, Lin WW (2005) Oregonin inhibits lipopolysaccharide-induced iNOS gene transcription and upregulates HO-1 expression in macrophages and microglia. Br J Pharmacol 146: 378-388.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences