AQP-3 in the Epidermis of Haemodialysis Patients with CKD-Associated Pruritus is Overexpressed
Akishi Momose, Tomihisa Funyu, Ryuichi Wada and Yasuo Shiraiwa
DOI10.21767/2472-5056.100007
Akishi Momose1,3*, Tomihisa Funyu1, Ryuichi Wada2 and Yasuo Shiraiwa3
1Department of Urology, Oyokyo Kidney Research Institute, Hirosaki Hospital, Japan
2Department of Pathology and Bioscience, Hirosaki Graduates School of Medicine, Japan
3Department of Urology, Jusendo General Hospital, Japan
- *Corresponding Author:
- Akishi Momose
Department of Urology
Jusendo General Hospital
1-1-17 Ekimae, Koriyama, Japan
Tel: +81-24-932-6363
E-mail: a.momose@jusendo.or.jp
Received date: April 10, 2016; Accepted date: April 27, 2016; Published date: April 29, 2016
Citation: Momose A, Funyu T, Wada R, Shiraiwa Y (2016) AQP-3 in the Epidermis of Haemodialysis Patients with CKD-Associated Pruritus is Overexpressed. J Clin Exp Nephrol 1: 7. DOI: 10.21767/2472-5056.100007
Copyright: © 2016 Momose A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Xerosis is considered to be one of the causes of chronic kidney disease-associated pruritus (CKD-aP). In the present study, the relationship between CKD-aP and aquaporin-3 (AQP-3), the water channel in the skin was examined in light of xerosis.
Methods: AQP-3 expression in the epidermis and patients characteristics included stratum corneum hydration (SCHy) were compared in haemodialysis patients (HD-pts) without pruritus (n = 23), HD-pts with slight to mild pruritus (n = 12), and HD-pts with moderate to severe pruritus (n = 7).
Results: In HD-pts, there was association between CKD-aP and both range and intensity of epidermal AQP-3 expression. The range and intensity of AQP-3 expression in the skin was significantly greater in HD-pts with severe CKD-aP than in HD-pts without CKD-aP, as shown by anti- AQP-3 staining that was both more intense and extended to more superficial layers of the skin.
Conclusions: CKD-aP was shown to be associated with epidermal AQP-3 expression but not with xerosis, which was quantified in this study using SC-Hy. In addition, there was no association between xerosis and epidermal AQP-3 expression. These results indicate that xerosis, which has been considered to be a cause of CKD-aP, is not a necessary and sufficient condition for CKD-aP, and that the mechanism of the onset of xerosis may not involve epidermal AQP-3 in HD-pts.
Keywords
Aquaporin-3 (AQP-3): Chronic kidney disease-associated pruritus (CKD-aP); Xerosis; Stratum corneum hydration (SC-Hy)
Introduction
Chronic kidney disease-associated pruritus (CKD-aP) is a very bothersome symptom in patients undergoing haemodialysis (HD-pts). The causes of CKD-aP have not yet been clarified. Many hypotheses have been suggested regarding the mechanisms underlying CKD-aP: secondary hyperparathyroidism, divalent-ion abnormalities [1], histaminergic dysfunction, proliferation of skin mast cells, irondeficiency anaemia, opioid system involvement, neuropathy and neurological changes, and xerosis (i.e., dry skin) [2]. Conflicting findings were reported by two studies that measured SC-Hy as an index of xerosis and examined its relationship with CKD-aP: the first found a relationship between SC-Hy and CKD-aP [3], while the second did not [4].
Thirteen human/mammalian aquaporins (AQP) have thus far been molecularly identified and localized to various epithelial, endothelial, and other tissues. Aquaporin-3 (AQP-3), which is permeable to water and glycerol, is the predominant aquaporin in the epidermis [5]. Hara et al. reported that lack of AQP-3 is related to a decrease in stratum corneum hydration (SC-Hy) [6]. Based on this finding, we hypothesized that the level of AQP-3 in the epidermis is lowered in HD-pts with CKDaP, causing dry skin and accompanying pruritus.
The aim of the current study was to investigate the involvement of epidermal AQP-3 in the relationship between CKD-aP and dry skin.
Subjects and Methods
This study received appropriate ethics committee approval from our local institutional review board in accordance with the Declaration of Helsinki. We conducted arteriovenous fistula surgeries on 68 HD-pts between June 2006 and December 2006 or between April 2015 and December 2015. Forty two patients (fourteen females and 28 males, 31–87 years old) agreed to participate in the study. Patients with concomitant atopic dermatitis or psoriasis were excluded. Haemodialysis was performed 4–5 hours three times per week using a high-flux membrane. We used heparin calcium (500 IU/ hour) as an anticoagulant. The degree of pruritus was classified according to the Shiratori‘s classification of itch in japan (Table 1). Based on the level of pruritus, subjects were divided into the following three groups: non-pruritus group (n=23), slight-to-mild pruritus group (n=12), moderate-tosevere pruritus group (n=7) and control group (n=3). Control group were the patients who had urological surgical operation (e.g., hypertrophy of prostate) and feel no pruritus. Subject characteristics, SC-Hy, and epidermal AQP-3 expression were compared between groups. SC-Hy was assessed using a skin moisture sensor, DM-R2 (Panasonic, Osaka, Japan) in the area of forearm.
| Score (severity) | Daytime symptoms | Night time symptoms |
|---|---|---|
| 4 (severe) | Intolerable itch not relieved by scratching but instead worsens. Cannot focus on work or study | Can hardly sleep because of itch. Scratching all the time, but itch intensifies with scratching. |
| 3 (moderate) | Scratching even in the presence of others. Irritation as a results of itch, continuous scratching | Wake up because of itch. Can fall asleep again after scratching, but continue to scratching un consciously while sleeping |
| 2 (mild) | Itch sensation is relieved by slight, occasional scratching. Not too disturbing. | Feel somewhat itchy, but can obtain relief by scratching. Do not wake up because of itch sensations. |
| 1 (slight) | Feel itchy sometimes, but tolerable without scratching. | Feel slightly itchy when going to sleep, but do not need to scratch. Sleeping well. |
| 0 (none) | Hardly feel itchy or do not feel itchy at all. | Hardly feel itchy or do not feel itchy at all. |
Table 1: Itch Classification (Shiratori’s classification).
Skin was obtained during vascular access operation or urological operation (control group) and immunostained with anti-AQP-3 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Immunostaining was performed using the streptavidin-biotin method. Paraffin sections were deparaffinized with xylene and ethanol, and endogenous peroxidase activity was blocked with 0.3% H2O2 in methanol. Sections were then microwaved in citrated buffer at pH 6.0 for 20 min. After incubation with 10% normal rabbit serum, sections were incubated overnight at 4°C with goat anti-AQP-3 antibody (Santa Cruz Biotechnology, 1:500). The sections were then incubated with biotinylated anti-goat immunoglobulin antibody and streptavidin-peroxide complex. Peroxidase activity was visualized with diaminobenzidine. The immunostaining was performed carefully under the same condition of incubation time and temperature. All measurements were performed in a controlled temperature room at 22 ± 1.0 and relative humidity of 40-50%
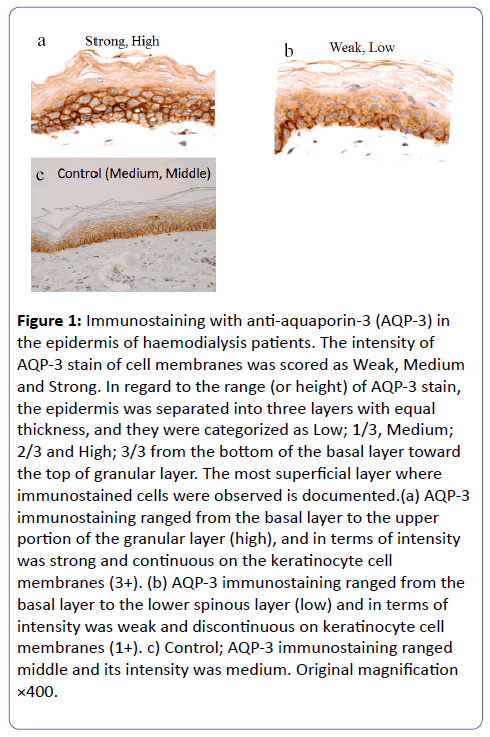
The expression of AQP-3 was evaluated with the vertical distribution in the epidermis and its intensity. The intensities were scored as weak, moderate and strong. The intensity score was the strongest expression in the skin. The epidermis was separated equally into three layers of low, middle, high from the bottom of the basal layer toward the top of the granular layer. The vertical distribution was scored as the highest layer, in which the expression of AQP3 was observed. In each skin specimen, three part of the epidermis was assessed with semiquantitative method. The expression of AQP-3 in the epidermis are shown in two cases with strong intensity and up to high layer, with weak intensity and to low layer typically and control with medium intensity and middle layer (Figure 1).
Figure 1: Immunostaining with anti-aquaporin-3 (AQP-3) in the epidermis of haemodialysis patients. The intensity of AQP-3 stain of cell membranes was scored as Weak, Medium and Strong. In regard to the range (or height) of AQP-3 stain, the epidermis was separated into three layers with equal thickness, and they were categorized as Low; 1/3, Medium; 2/3 and High; 3/3 from the bottom of the basal layer toward the top of granular layer. The most superficial layer where immunostained cells were observed is documented.(a) AQP-3 immunostaining ranged from the basal layer to the upper portion of the granular layer (high), and in terms of intensity was strong and continuous on the keratinocyte cell membranes (3+). (b) AQP-3 immunostaining ranged from the basal layer to the lower spinous layer (low) and in terms of intensity was weak and discontinuous on keratinocyte cell membranes (1+). c) Control; AQP-3 immunostaining ranged middle and its intensity was medium. Original magnification ×400.
Statistical analysis
All values are expressed as means ± standard deviations (SD) for normally distributed data, median (range) for nondistributed data, and number (percentages). Statistical analysis was performed using Stat View version 5.0 software (Abacus Concepts, Inc., Berkeley, CA). Results were analysed using nonrepeated measures ANOVA, the Kruskal-Wallis H-test, or Yates m×n chi-square test for categorical outcomes. Significant differences found by ANOVA or the Kruskal-Wallis H-test was further confirmed by Steel and Steel-Dwass methods. P-values <0.05 were considered to be statistically significant.
Results
Patient characteristics are presented in Table 2. There were no significant differences between the three groups in terms of the following characteristics: age, sex, primary disease, duration of haemodialysis, presence or absence of hepatitis B and hepatitis C, presence or absence of antiallergic drug or ointment use, calcium level, phosphorus level, intact parathyroid hormone level, albumin level, serum C-reactive protein (CRP) level, or Kt/V (total urea clearance x time on dialysis/total body water).
| Non-Pruritus | Pruritus (slight-mild) | Pruritus (moderate-severe) | p | |
|---|---|---|---|---|
| n | 23 | 12 | 7 | |
| Age(y.o.) | 68.1±12.4 | 68.4±14.9 | 70.1±11.0 | n.s |
| Sex (F/M) | 8/15 | 3/9 | ¾ | n.s |
| Orignal disease (DM/CGN/HT/Unknown) | 13/7/2/1 | 10/1/1/0 | 3/3/1/0 | n.s |
| Duration of HD(m) | 3 (1-242) | 19 (1-96) | 5 (1-252) | n.s |
| HBV/HCV | 3 | 0 | 2 | n.s |
| Use of anti-allergy drug or ointment | 6 (26%) | 3 (25%) | 3(43%) | n.s |
| Corrected Ca (mg/dl) | 9.0±1.2 | 9.2±0.2 | 8.4±0.4 | n.s |
| iP (mg/dl) | 5.4±1.6 | 5.0±1.5 | 4.6±1.3 | n.s |
| i-PTH (pg/ml) | 133(55-282) | 214(42-412) | 160(116-578) | n.s |
| Serum Alb (g/dl) | 3.5±0.7 | 3.1±0.5 | 3.1±0.4 | n.s |
| CRP (mg/dl) | 0.44(0.11-8.0) | 0.13(0.02-1.71) | 0.34(0.08-6.3) | n.s |
| Sp Kt/V | 1.1±0.5 | 1.2±0.2 | 1.1±0.5 | n.s |
| Stratiumcorneum hydration (%) | 33.0±1.5 | 32.4±1.3 | 32.7±0.6 | n.s |
Table 2: Patient Characteristics; Values are presented as mean ± SD, median (range), or number of patients. Values are presented as mean ± SD, median (range), or number of patients.DM, diabetes mellitus; CGN, chronic glomerular nephritis; HT, hypertension; iP, inorganic phosphorus; sp Kt/V, single-pool model Kt/V.
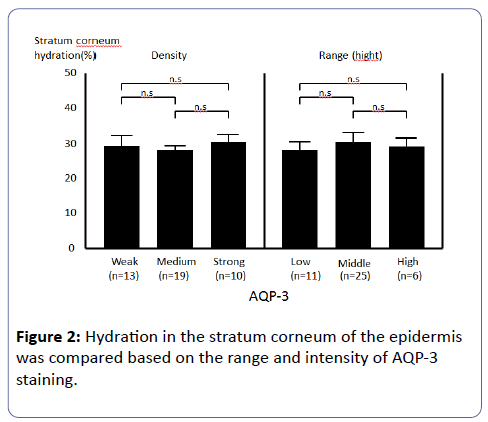
SC-Hy did not differ significantly between the three groups either. When SC-Hy and AQP-3 in the skin of HD-pts were examined, no significant correlation was observed between SC-Hy and either intensity or range of epidermal AQP-3 expression (Figure 2). The range and intensity of AQP-3 expression in the skin was greater in HD-pts with severe CKDaP than in those without CKD-aP, as shown by staining that occurred over a wider range (up to the surface layer of the skin) and at higher intensity (Tables 3a and 3b). There is no significant correlation between the levels of parathyroid hormones, calcium (Ca), phosphorus (P), Ca×P products in the blood with intensity or range of epidermal AQP-3 expression in the epidermis.
| Control | Non-pruritus* | Pruritus*,** (slight-mild) | Pruritus*,** (severe-moderate) | |
|---|---|---|---|---|
| (n=3) | (n=23) | (n=12) | (n=7) | |
| Weak | 1 (33%) | 13 (57%) | 1 (8%) | 0 (0%) |
| Medium | 2 (67%) | 10 (43%) | 5 (42%) | 3 (43%) |
| Strong | 0 (0%) | 0 (0%) | 6 (50%) | 4 (57%) |
Table 3a: Intensity of AQP-3 expression.
| Control | Non-pruritus* | Pruritus*,** (slight-mild) | Pruritus*,** (severe-moderate) | |
|---|---|---|---|---|
| (n=3) | (n=23) | (n=12) | (n=7) | |
| Low | 0 (0%) | 11 (48%) | 0 (0%) | 0 (0%) |
| Middle | 3(100%) | 12 (52%) | 9 (75%) | 4 (57%) |
| High | 0 (0%) | 0 (0%) | 3 (25%) | 3 (43%) |
Table 3b: Range of AQP-3 expression; The epidermis was separated equally into three layers of low, middle, high from the bottom of the basal layer toward the top of the granular layer. The range (i.e., the vertical distribution) was scored as the highest layer, in which the expression of AQP3 was observed. *; p<0.01 vs Control Group, **; p<0.01 vs Non- Pruritus group, The Kruskal-Wallis H-test,the Steel and Steel- Dwass methods
Discussion
Xerosis is considered to be one of the causes of CKD-aP [2]. In one study, CKD-aP was observed in 77% of HD-pts with moderate to severe xerosis and 39% of those without xerosis [7]. In another study of HD-pts with severe CKD-aP, 34% of subjects had accompanying xerosis while 21% did not [8]. Thus, there is indeed a tendency toward a higher incidence of CKD-aP in HD-pts with xerosis, but xerosis does not seem to be a necessary and sufficient condition for CKD-aP. In our study, no association was noted between CKD-aP and SC-Hy. Xerosis is a condition that is clinically diagnosed based on inspection and palpation, and should in principle be accompanied by reduced SC-Hy. However, in a strict sense, the two might not occur concurrently because conditions not marked by reduced SC-Hy can be clinically diagnosed as xerosis due to the impact of keratinization, hardness of the skin, and the thickness of the epidermis.
In healthy humans, 13 types of aquaporin (AQP-0 to -12) have been identified thus far. In the skin, AQP-3 is mainly found in the basal layer of the epidermis. AQP-3, also called aquaglyceroporin, serves as a channel that is permeable not only to water but also to glycerol and urea [9]. Diseases that present with dry skin include atopic dermatitis, eczema, psoriasis, senile xerosis, and hereditary ichthyosis. Among these, senile xerosis is considered to be caused by reduced skin AQP-3 and insufficient transport of water in the skin [10]. In contrast, the xerosis observed in atopic dermatitis is thought to develop when increased AQP-3 in the skin accelerates the transport of water in the epidermis, increasing insensible perspiration from the surface of the skin (i.e., TEWL) [11]. However, we did not find any significant correlation between xerosis (i.e., SC-Hy) and either the intensity or range of epidermal AQP-3 expression in HD-pts. Therefore, our results showed that the onset of xerosis in HD-pts is not determined by the degree of epidermal AQP-3 expression, as is the case with xerosis in atopic dermatitis or senile xerosis. Yosipovitch et al. suggested that the abnormal stratum corneum integrity and low levels of glycerol in end-stage renal disease (ESRD) could be associated with low levels of AQP3, as AQP3 transports glycerol in the skin [12]. The suggestion of Yosipovitch group resembles the mechanism of senile xerosis that is considered to be caused by reduced skin AQP-3 and insufficient transport of water in the skin [10]. Contrary to the suggestion of Yosipovitch group, our results resembles the mechanism of the xerosis observed in atopic dermatitis that is thought to develop when increased AQP-3 in the skin accelerates the transport of water in the epidermis, increasing TEWL[11]. However, the decrease of SC-Hy observed in atopic dermatitis was not recognized in HD-pts with and without CKD-aP.
Staining representing epidermal AQP-3 expression was more intense and covered a wider area (up to the upper squamous cell layer) in HD-pts with severe CKD-aP than in HD-pts without CKD-aP. The conditions that have been shown to demonstrate increased epidermal AQP-3 expression include inflammation [13,14], increased retinoic acid [15], elevated pH [16], exposure to ultraviolet light [17], increased osmotic pressure [18], healing during skin disorders [19], squamous cell carcinoma [20], and increased epidermal growth factor [21]. Moreover, those conditions are associated with pruritus [22-25]. Though there was no significant difference of serum CRP levels between the three groups, it has been confirmed that levels of CRP are elevated in HD-pts with CKD-aP [26]. AQP-3 expression in HD-pts with CKD-aP may be intensified due to inflammation. Intensified AQP-3 expression can indicate increased expression of channels other than AQP-3 (e.g., calcium channels) [1,27], which leads to the production of pruritogens by keratinocytes [28].
In conclusion, epidermal AQP-3 expression was intensified and occurred over an extended area, up to the upper squamous cell layer, in HD-pts with accompanying CKD-aP.
References
- Momose A, Kudo S, Sato M, Saito H, Nagai K, et al. (2004) Calcium ions are abnormally distributed in the skin of haemodialysis patients with uraemic pruritus. Nephrol Dial Transplant 19: 2061-2066.
- Nordal EJ, Os I (2007) [Uremic pruritus--pathogenesis and treatment]. Tidsskr Nor Laegeforen 127: 1201-1203.
- Morton CA, Lafferty M, Hau C, Henderson I, Jones M, et al. (1996) Pruritus and skin hydration during dialysis. Nephrol Dial Transplant 11: 2031-2036.
- Yosipovitch G, Tur E, Morduchowicz G, Boner G (1993) Skin surface pH, moisture, and pruritus in haemodialysis patients. Nephrol Dial Transplant 8: 1129-1132.
- Sougrat R, Morand M, Gondran C, Barré P, Gobin R, et al. (2002) Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J Invest Dermatol 118: 678-685.
- Hara M, Ma T, Verkman AS (2002) Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J BiolChem 277: 46616-46621.
- Balaskas EV, Chu M, Uldall RP, Gupta A, Oreopoulos DG (1993) Pruritus in continuous ambulatory peritoneal dialysis and hemodialysis patients. Perit Dial Int 13 Suppl 2: S527-532.
- Szepietowski JC, Reich A, Schwartz RA (2004) Uraemic xerosis. Nephrol Dial Transplant 19: 2709-2712.
- Rojek A, Praetorius J, Frøkiaer J, Nielsen S, Fenton RA (2008) A current view of the mammalian aquaglyceroporins. Annu Rev Physiol 70: 301-327.
- Dumas M, Sadick NS, Noblesse E, Juan M, Lachmann-Weber N, et al. (2007) Hydrating skin by stimulating biosynthesis of aquaporins. J Drugs Dermatol 6: s20-24.
- Olsson M, Broberg A, Jernås M, Carlsson L, Rudemo M, et al. (2006) Increased expression of aquaporin 3 in atopic eczema. Allergy 61: 1132-1137.
- Yosipovitch G, Duque MI, Patel TS, Ishiuji Y, Guzman-Sanchez DA, et al. (2007) Skin barrier structure and function and their relationship to pruritus in end-stage renal disease. Nephrol Dial Transplant 22: 3268-3272.
- Tancharoen S, Matsuyama T, Abeyama K, Matsushita K, Kawahara K, et al. (2008) The role of water channel aquaporin 3 in the mechanism of TNF-alpha-mediated proinflammatory events: Implication in periodontal inflammation. J Cell Physiol 217: 338-349.
- Marchini G, Ståbi B, Kankes K, Lonne-Rahm S, Østergaard M, et al. (2003) AQP1 and AQP3, psoriasin, and nitric oxide synthases 1-3 are inflammatory mediators in erythema toxicumneonatorum. PediatrDermatol 20: 377-384.
- Bellemère G, Von Stetten O, Oddos T (2008) Retinoic acid increases aquaporin 3 expression in normal human skin. J Invest Dermatol 128: 542-548.
- Dumas M, Gondran C, Barre P, et al. Effect of an Ajugaturkestanica extract on aquaporin 3 expression, water flux, differentiation and barrier parameters of the human epidermis. Eur J Dermatol 2002; 12(6): 25-26.
- Natarajan VT, Ganju P, Ramkumar A, Grover R, Gokhale RS3 (2014) Multifaceted pathways protect human skin from UV radiation. Nat ChemBiol 10: 542-551.
- Sugiyama Y, Ota Y, Hara M, Inoue S (2001) Osmotic stress up-regulates aquaporin-3 gene expression in cultured human keratinocytes. BiochimBiophysActa 1522: 82-88.
- Cao C, Sun Y, Healey S, Bi Z, Hu G, et al. (2006) EGFR-mediated expression of aquaporin-3 is involved in human skin fibroblast migration. Biochem J 400: 225-234.
- Hara-Chikuma M, Verkman AS (2008) Prevention of skin tumorigenesis and impairment of epidermal cell proliferation by targeted aquaporin-3 gene disruption. Mol Cell Biol 28: 326-332.
- Huang Y, Zhu Z, Sun M, et al. Critical role of aquaporin-3 in the human epidermal growth factor-induced migration and proliferation in the human gastric adenocarcinoma cells. Cancer Biology & Therapy. 2010; 9(12): 1000-7.
- Kimmel M, Alscher DM, Dunst R, Braun N, Machleidt C, et al. (2006) The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant 21: 749-755.
- Berne B, Vahlquist A, Fischer T, Danielson BG, Berne C (1984) UV treatment of uraemic pruritus reduces the vitamin A content of the skin. Eur J Clin Invest 14: 203-206.
- de Kroes S, Smeenk G (1983) Serum vitamin A levels and pruritus in patients on hemodialysis. Dermatologica 166: 199-202.
- Richardson C, Upton D, Rippon M (2014) Treatment for wound pruritus following burns. J Wound Care 23: 227-228, 230, 232-3.
- Chen HY, Chiu YL, Hsu SP, Pai MF, Lai CF, et al. (2010) Elevated C-reactive protein level in hemodialysis patients with moderate/severe uremic pruritus: a potential mediator of high overall mortality. QJM 103: 837-846.
- Ikarashi N, Ogiue N, Toyoda E, Kon R, Ishii M, et al. (2012) Gypsum fibrosum and its major component CaSO4 increase cutaneous aquaporin-3 expression levels. J Ethnopharmacol 139: 409-413.
- Bollag WB, Xie D, Zheng X, Zhong X (2007) A potential role for the phospholipase D2-aquaporin-3 signaling module in early keratinocyte differentiation: production of a phosphatidylglycerol signaling lipid. J Invest Dermatol 127: 2823-2831.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences